DANGERS of Mixing CLORINE
advertisement

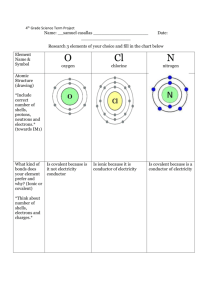
DANGERS of Mixing CLORINE (CLOROX) & AMMONIA ***It will make Chlorine Gas (among other deadly things) That warning is there to protect you. Household bleach has a chemical formula of NaOCl - that is, one atom each of sodium, oxygen, and chlorine. Its chemical name, for the curious, is sodium hypochlorite. Ammonia has a chemical formula of NH3, that is, one atom of nitrogen and three atoms of hydrogen. When these two compounds are combined, the following reaction takes place: 2(parts)NaOCl + 2NH3 --> 2NaONH3 + Cl2. Do you see that Cl2 on the right hand side there? This means one part chlorine gas, made up of diatomic (two atom) molecules. It also means that the chlorine gas has been liberated from the bleach, and is quite capable of causing you harm when inhaled! ***Nitrogen Trichloride (very toxic) Another potential reaction, which occurs when a greater amount of bleach is added than ammonia, is this: 3NaOCl + NH3 --> 3NaOH + NCl3 That's sodium hydroxide and nitrogen trichloride. Nitrogen trichloride is a very toxic chemical to humans, and even if you did get close enough to ingest it, it would probably explode in your face first, as it is also a very volatile explosive!! There is little necessity in explaining why that is bad. ***Hydrazine (explosive)…rocket fuel) Another reaction can occur if you have more ammonia than bleach. It is a three-part process. The first reaction produces sodium hydroxide and chlorine These products react with more ammonia. NH3 + NH2Cl + NaOH -->N2H4 + NaCl + H2O. N2H4 is hydrazine, a very unstable compound (like some of U ). Then a final reaction between hydrazine and dichloramine occurs. 2NH2Cl + N2H4 --> 2 NH4Cl + N2. This reaction produces so much heat that it usually causes an explosion!!!