Chapter 7 Vocabulary

Glossary
Chapter 7 absolute zero The lowest possible temperature; the temperature at which the particles in a material, atoms or molecules, contain no energy of motion that can be extracted from the body. absorption line A dark line in a spectrum; produced by the absence of photons absorbed by atoms or molecules. absorption spectrum
(dark-line spectrum)
A spectrum that contains absorption lines.
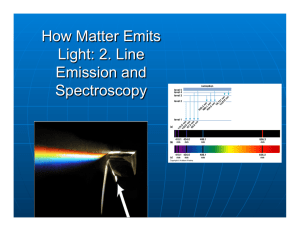
Balmer series Spectral lines in the visible and near-ultraviolet spectrum of hydrogen produced by transitions whose lowest orbit is the second. binding energy The energy needed to pull an electron away from its atom. black body radiation blue shift
Radiation emitted by a hypothetical perfect radiator; the spectrum is continuous, and the wavelength of maximum emission depends only on the body's temperature.
The shortening of the wavelengths of light observed when the source and observer are approaching each other. collisional broadening continuous spectrum
The smearing out of a spectral line because of collisions among the atoms of the gas.
A spectrum in which there are no absorption or emission lines.
Coulomb force The repulsive force between particles with like electrostatic charge. density The amount of matter per unit volume in a material; measured in grams per cubic centimeter, for example.
Doppler broadening
The smearing of spectral lines because of the motion of the atoms in the gas.
Doppler effect The change in the wavelength of radiation due to relative radial motion of source and observer. electron Low-mass atomic particle carrying a negative charge. emission line A bright line in a spectrum caused by the emission of photons from atoms. emission spectrum
(bright-line spectrum)
A spectrum containing emission lines. energy level One of a number of states an electron may occupy in an atom, depending on its binding energy. excited atom An atom in which an electron has moved from a lower to a higher orbit. ground state The lowest permitted electron orbit in an atom. ion ionization
An atom that has lost or gained one or more electrons.
The process in which atoms lose or gain electrons.
Atoms that have the same number of protons but a different number of neutrons. isotopes
Kelvin temperature scale
The temperature, in Celsius (centigrade) degrees, measured above absolute zero.
Kirchhoff's laws
A set of laws that describes the absorption and emission of light by matter.
L dwarf A type of star that is even cooler than the M stars.
Lyman series Spectral lines in the ultraviolet spectrum of hydrogen produced by transitions whose lowest orbit is
molecule neutron the ground state.
Two or more atoms bonded together.
An atomic particle with no charge and about the same mass as a proton. nucleus (of an atom)
The central core of an atom containing protons and neutrons; carries a net positive charge.
Paschen series
Spectral lines in the infrared spectrum of hydrogen produced by transitions whose lowest orbit is the third. permitted orbit One of the energy levels in an atom that an electron may occupy. proton A positively charged atomic particle contained in the nucleus of atoms; the nucleus of a hydrogen atom. quantum mechanics radial velocity
(V r
) red shift
The study of the behavior of atoms and atomic particles.
That component of an object's velocity directed away from or toward Earth.
The lengthening of the wavelengths of light seen when the source and observer are receding from each other.
A star's position in the temperature classification system O, B, A, F, G, K, and M. Based on the appearance of the star's spectrum. spectral class or type spectral sequence
T dwarf
The arrangement of spectral classes (O, B, A, F, G, K, M) ranging from hot to cool.
A very-low-mass star at the bottom end of the main sequence with a cool surface and a low luminosity. temperature A measure of the velocity of random motions among the atoms or molecules in a material. thermal energy The energy stored in an object as agitation among its atoms and molecules. transition The movement of an electron from one atomic orbit to another. wavelength of maximum intensity
The wavelength at which a perfect radiator emits the maximum amount of energy; depends only on the object's temperature.