Unit 6: Chemical Formulas
advertisement
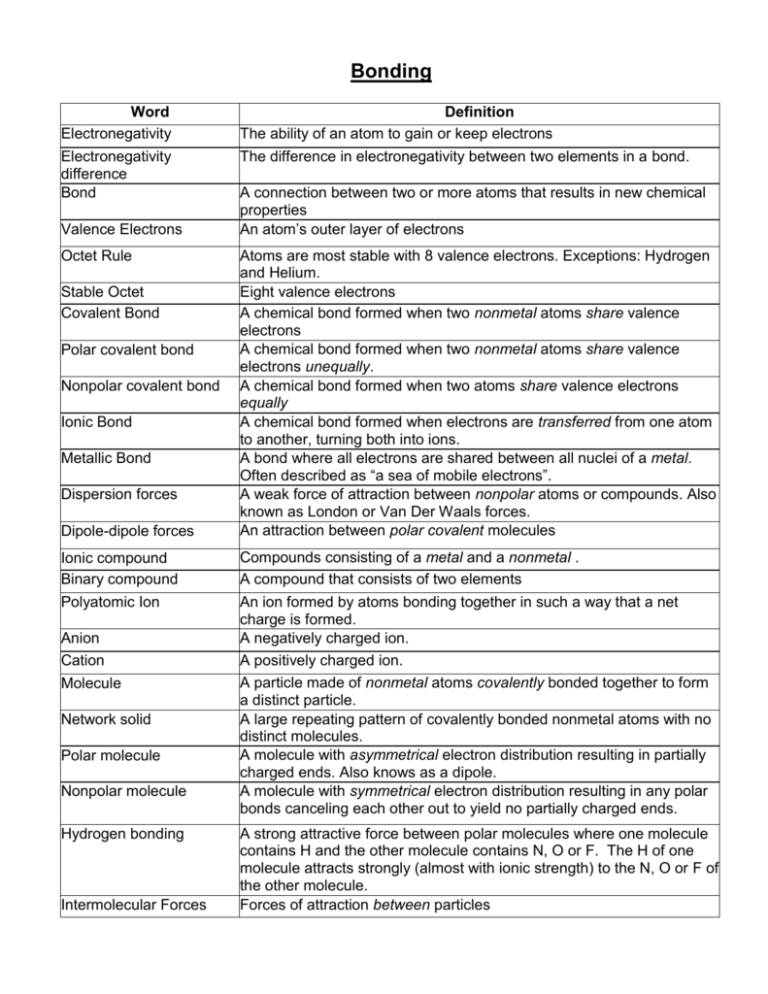
Bonding Word Electronegativity Definition The ability of an atom to gain or keep electrons Electronegativity difference Bond The difference in electronegativity between two elements in a bond. Valence Electrons Octet Rule Stable Octet Covalent Bond Polar covalent bond Nonpolar covalent bond Ionic Bond Metallic Bond Dispersion forces Dipole-dipole forces Ionic compound Binary compound Polyatomic Ion Anion Cation Molecule Network solid Polar molecule Nonpolar molecule Hydrogen bonding Intermolecular Forces A connection between two or more atoms that results in new chemical properties An atom’s outer layer of electrons Atoms are most stable with 8 valence electrons. Exceptions: Hydrogen and Helium. Eight valence electrons A chemical bond formed when two nonmetal atoms share valence electrons A chemical bond formed when two nonmetal atoms share valence electrons unequally. A chemical bond formed when two atoms share valence electrons equally A chemical bond formed when electrons are transferred from one atom to another, turning both into ions. A bond where all electrons are shared between all nuclei of a metal. Often described as “a sea of mobile electrons”. A weak force of attraction between nonpolar atoms or compounds. Also known as London or Van Der Waals forces. An attraction between polar covalent molecules Compounds consisting of a metal and a nonmetal . A compound that consists of two elements An ion formed by atoms bonding together in such a way that a net charge is formed. A negatively charged ion. A positively charged ion. A particle made of nonmetal atoms covalently bonded together to form a distinct particle. A large repeating pattern of covalently bonded nonmetal atoms with no distinct molecules. A molecule with asymmetrical electron distribution resulting in partially charged ends. Also knows as a dipole. A molecule with symmetrical electron distribution resulting in any polar bonds canceling each other out to yield no partially charged ends. A strong attractive force between polar molecules where one molecule contains H and the other molecule contains N, O or F. The H of one molecule attracts strongly (almost with ionic strength) to the N, O or F of the other molecule. Forces of attraction between particles Bonding *Atoms gain, lose, or share electrons to achieve a full outer valence layer and become more stable* A. Elements join to form compounds with different chemical and physical properties 1. Dependent on elements involved (metal, nonmetal) and the type of bond formed (ionic, covalent, metallic) B. Compounds contain elements chemically combined in a fixed proportion (ex: ammonia always contains a 1:3 ratio of nitrogen atoms to hydrogen atoms) and can only be broken apart by chemical means C. Compounds have specific chemical formulas, and can be named according to IUPAC rules D. Physical properties are dependent on chemical bonds and intermolecular forces. These properties include malleability, solubility, conductivity, hardness, melting point, and boiling point E. There are three types of bonding 1. Ionic – electrons transferred (between a metal and a nonmetal) - note: ionic compounds which contain polyatomic ions will have both covalent and ionic bonding 2. Covalent (Nonmetals)– electrons shared Two different nonmetals (ex: H-O) = Polar bond The same nonmetals (ex: H-H) = Non-polar bond 3. Metallic (metals) – mobile “sea of valence electrons” shared by all atoms G. Atoms can gain or lose electrons to become ions 1. An anion is an atom that has gained one or more electrons, thus obtaining a negative charge and a larger radius 2. A cation is an atom that has lost one or more electrons, thus obtaining a positive charge and a smaller radius H. Lewis Electron Dot Diagrams can be used to represent ionic and covalent bonding by showing the arrangement of valance electrons I. Atoms bond with one another to release energy, obtaining a stable valance electron configuration. 1. The noble gases with a full valance shell are already stable. J. How strongly an atom attracts the electrons in a chemical bond is indicated by its electronegativity 1. Electronegativity is the ability of an atom to attract electrons to itself K. The difference in electronegativity between two bonded atoms determines the degree of polarity of the bond. 1. The greater the electronegativity difference the greater the polarity of the bond L. Molecular polarity can be determined by the shape of the molecule and the distribution of charge. 1. Symmetrical molecules are non-polar because electronegativity differences cancel each other out 2. Asymmetrical molecules are polar because of the difference in electronegativity values M. There are three main types of intermolecular forces 1. Hydrogen bonding is created by the unequal distribution of charge between the hydrogen atom in one molecule with an extremely electronegative element in another molecule (N,O,F). a) Strongest IMF highest melting and boiling points 2. Dipole-Dipole forces are the attraction between oppositely charged ends of neighboring polar molecules a) The greater the polarity the stronger the IMF 3. Dispersion forces (also called London, or Van Der Waals) are weak forces between nonpolar molecules a) Weakest IMF lowest melting and boiling points N. The different types of bonding influence the properties of materials 1. Ionic Solid - Force of attraction is quite strong! - High melting point due to high temp. needed to overcome both inter- and intra- molecular bonds - Poor conductors of electricity - except in the liquid state when the ions are free to move around 2. Molecular Solid - Relatively soft, low melting point due to weak intermolecular forces that are easy to overcome. - Good insulators - Does not conduct electricity due to no free moving electrons 3. Metallic Crystals - Good conductors of electricity - Malleable and ductile – the attraction between the nuclei and electrons holds the metal crystal together 4. Covalent Network Solids - Very hard, high melting points, nonconductors of electricity. - Share e- but compressed so much that there is a network like diamond - Complex – very strong O. Energy always plays a role in simple chemical reactions 1. Chemical changes result in the shift of energy a.) The formation of bonds is exothermic – energy is released (more stable) b.) The breaking of bonds is endothermic – energy is absorbed (less stable) P. Energy, mass and charge are all conserved in every chemical reaction. “Laws of Conservation” Caution: There is a difference between BOND Polarity (Classification) and MOLECULE polarity (Classification) Naming review: [name of Cation] [name of Anion] change ending to –ide Note: Polyatomic ions keep their names as is. (see Table E) IONIC COVALENT Does the cation have more than one possible oxidation state? NO YES Use prefixes (mono, di, tri, tetra) to indicate multiple atoms of the same element. Note: No prefix is needed on the cation if there is only 1 of them. name is complete name of the compound must include the oxidation state in (roman numerals) immediately following the element. 1) IONIC BONDING HOMEWORK 1) Draw a dot diagram of an ion of potassium ionically bonding to an ion of chlorine, to make potassium chloride (KCl). 2) Draw a dot diagram of an ion of calcium ionically bonding to two atoms of fluorine, to make calcium fluoride (CaF2). 3) Draw a dot diagram of two ions of sodium ionically bonding to an ion of oxygen (oxide), to make sodium oxide (Na2O). 4) Which species will conduct electricity (is an electrolyte)? a) NaCl (s) b) N2 (s) c) LiF (aq) d) CaI2 (s) 5) Explain how you determined the answer to #4. 6) Which species will form an anion when forming an ionic bond? a) O b) Li c) S 7) Explain your answer to #6. 8) Why will Al+3 and O-2 attract while Al+3 and Fe+2 repel? 9) What is the charge of an element before it forms a bond? Why? d) Sr Topic 2) Properties of Ionic Substances Homework 1) Which substance has a high melting point and conducts electricity when dissolved in water? a) Mg b) KBr c) CH4 d) SiO2 2) List one experiment that can determine whether a substance is ionic or not: 3) What would the expected outcome of the experiment be if the substance was ionic? 4) Complete the following chart: Ion Formula Ion Name Cu+1 Au+3 Cl-1 N-3 K+1 Cs+1 Cr+3 Ag+1 Ion Name Gold (I) Zinc Phosphide Bromide Tin (IV) Scandium Aluminum Lead (II) Topic 3) Ionic Names and Formulas Homework Formula NaCl Na2O H2SO4 Fe3(PO4)2 HBr Name Ion Formula CaSO4 K2O ZnSO3 (NH4)2SO4 HCH3COO PbCl2 CuSO4 Fe2S3 AgNO2 Name Lead (IV) chloride Potassium oxide Sodium phosphate Ammonium nitrate Lead (IV) sulfide Silver chloride Zinc sulfate Aluminum iodide Magnesium hydroxide Tin (II) acetate Ammonium oxide Potassium permanganate Iron (III) chloride Sodium cyanide Formula Topic 4: Covalent Bonding Homework 1) Complete the following chart, drawing the dot diagram of each element in the molecule and then the dot diagram of the molecule. If the formula is H2O, make sure you have two atoms of H and one of O in your dot diagram of the molecule. Formula Dot diagram for: Dot diagram for: F2 F F HBr H Br Dot Diagram of Molecule H O N H H2O NH3 2) Identify the following bonds as being polar covalent or nonpolar covalent. For the polar covalent bonds, label the + and - ends. Bond Polar or Nonpolar? If polar, label the + and - ends H–H H–H H–C H–C H – Cl H – Cl C – Cl C – Cl P – Cl P – Cl Cl – Cl Cl – Cl H–P H–P H–O H–O O–O O–O 3) When two atoms of nitrogen bond, how many pairs of electrons will be shared between them? a) 1 b) 2 c) 3 d) 4 4) When two atoms of fluorine bond, how many electrons will be shared between them? a) 1 b) 2 c) 3 d) 4 5) When an atom of H and an atom of F bond together: a) The H will be partially positive, because it has higher electronegativity than F. b) The H will be partially negative, because it has higher electronegativity than F. c) The F will be partially positive, because it has higher electronegativity than H. d) The F will be partially negative, because it has higher electronegativity than H. 6) Which of the molecules listed below has the most polar bond between the bonded atoms, in terms of greatest END? a) HF b) HCl c) HBr d) HI 7) Explain, in terms of electronegativity difference, why Cl2 contains nonpolar covalent bonds. 8) Explain, in terms of electronegativity difference, why H2O contains polar covalent bonds. Topic 5: Covalent Compounds Homework 1) For each of the following molecules represented by structural formulas indicate a) if the molecule is polar or nonpolar. b) If polar, label which side is partially positive and which side is partially negative. c) Identify the shape of the molecule d) identify the type of attractive force that will hold molecules of this substance together in the liquid and solid phase. e) Name the molecule If nonpolar, b) and c) do not get done. Molecule (do B here) Polar or Nonpolar Shape H – Cl H–H H–O | H H-N–H | H Cl | Cl – C – Cl | Cl O=C=O H - Br IMF Type Name 2) As a general rule, the boiling point of a molecular substance increases as the molecular mass increases. Create a graph with molecular mass on the X axis and the boiling point on the Y axis. Start temperature at 100K and molecular mass at 0 grams/mole. Plot the data below: Substance Molecular Mass (grams/mole) Boiling Point (K) H2S 34 211 H2Se 81 229 H2Te 130 271 a) H2O has a molecular mass of 18 g/mol. Extrapolate the curve and estimate the boiling point of water, putting an X in a circle on the extrapolated line at 18 g/mol. b) Water actually boils at 373 K. Put an “A” where the actual boiling point of water is, at 18 g/mol, NOT on the extrapolated line. c) Is the actual boiling point of water higher or lower than the extrapolated estimate? d) Explain why this difference exists in terms of the specific kind of attractive force that holds water molecules to each other in the solid and liquid phase. 3) Identify the type of compound indicated in the table of properties below and explain how you arrived at that determination (these include metallic and ionic, from previous units): Compound A B Melting Point 1074 K 42 K Boiling Point 2556 K 88 K Electrical Conductivity (s) No No Electrical Conductivity (l) Yes No C 303 K 2676 K Yes Yes D 3820 K 5100 K No No A_______________________________________________ Why: B_______________________________________________ Why: C_______________________________________________ Why: D_______________________________________________ Why:
![QUIZ 2: Week of 09.03.12 Name: [7pts] 1.) Thoughtful list of 3](http://s3.studylib.net/store/data/006619037_1-3340fd6e4f1f4575c6d8cf5f79f0ff3e-300x300.png)





