4. The molecular formulae of some compounds that can be prepared
advertisement

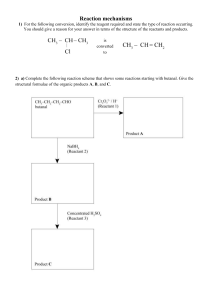
4. The molecular formulae of some compounds that can be prepared from ethanoic acid are given in the scheme below. C 2 H 4 O2 ethanoic acid ethanol/conc H 2 SO4 /heat C4 H 8O 2 P (a) (i) C 2 H 3 O 2 Na Q PCl 5 C 2 H 3 OCl R C 2 H 5 ON S Give the name and graphical formula of P. Name................................................................................................................. Structural formula (2) (ii) Give the name of the type of reaction which occurs when P is formed from ethanoic acid. ........................................................................................................................... (1) (b) Ethanoic acid can be obtained from P. (i) Give the name of the reagent(s) and state the conditions required. ........................................................................................................................... ........................................................................................................................... (2) (ii) Write a balanced equation for the reaction ........................................................................................................................... (1) (c) (i) State the reagent and reaction conditions that could be used for converting ethanoic acid into Q. ........................................................................................................................... ........................................................................................................................... (2) (ii) Give the name and graphical formula of the organic product of the reaction between anhydrous samples of Q and R. Name................................................................................................................. Structural formula (2) (iii) State how the product formed in (c)(ii) could be converted into ethanoic acid and write an equation for the reaction. ........................................................................................................................... ........................................................................................................................... (2) (d) (i) Give the name and graphical formula of the amide S. Name........................................................................................................ Structural formula................................................................................... (2) (ii) State the reagent(s) and reaction conditions that could be used for converting ethanoic acid into S. ........................................................................................................................... ........................................................................................................................... (2) (iii) Write a balanced equation for the reaction between S and aqueous hydrochloric acid. ........................................................................................................................... (1) (Total 17 marks) 5. The figure shows a reaction scheme for some aromatic compounds. CH 3 benzene compound P concentrated H 2 SO4 / concentrated HNO 3 at 55 ºC NO 2 Sn/HCl(aq) (a) (i) compound Q nitrobenzene Give the reagents and conditions for the conversion of benzene into compound P. ........................................................................................................................... ........................................................................................................................... (3) (ii) Give the name of the mechanism of this reaction. ........................................................................................................................... (2) (b) Draw the structural formulae of the possible organic products when excess chlorine is passed through boiling compound P in strong sunlight. (3) (c) (i) Classify the type of reaction occurring when nitrobenzene is converted into compound Q. ........................................................................................................................... (1) (ii) Draw the graphical formula of compound Q. (2) (d) Classify the types of reaction and draw the graphical formulae of the organic products of the reaction of propanal with: (i) sodium tetrahydridoborate(III), NaBH4; Type of reaction ............................................................................................... (2) (ii) Fehling’s solution; Type of reaction ............................................................................................... (2) (iii) hydrogen cyanide. Type of reaction ............................................................................................... (2) (Total 17 marks) 6. (a) Draw the structure of each of the three ketones which have the molecular formula C5H10O. For each compound give the ratio of the areas under each peak in its low-resolution proton n.m.r. spectrum. (6) (b) Draw the structure of each of the four aldehydes which also have the molecular formula C5H10O. Label with the letter X the compound which has only two peaks in its low-resolution proton n.m.r. spectrum. Label with the letter Y the compound which has five peaks with the ratios of the areas under each peak 3:3:2:1:1 in its low-resolution proton n.m.r. spectrum. Label with the letter Z the compound which shows optical isomerism. (7) (c) When carbonyl compounds react with HCN, racemic mixtures are often produced. Name the type of mechanism involved and explain what is meant by the term racemic mixture. Choose any carbonyl compound which does not form a racemic mixture when it reacts with HCN and draw the structure of the product formed by the reaction of this carbonyl compound with HCN. (4) (d) Explain why aldehydes react with Tollen’s (or Fehling’s) reagent but ketones do not. (3) (Total 20 marks) 7. N-Phenylethanamide can be prepared from benzene in three steps: NH 2 NO 2 Step 1 (a) Step 2 NHCOCH 3 Step 3 Give the reagents required to carry out Step 1 and write an equation for the formation of the reactive inorganic species present. Name and outline the mechanism for the reaction between this species and benzene. Reagents...................................................................................................................... Equation for formation of reactive species................................................................. ..................................................................................................................................... Name of mechanism.................................................................................................... Mechanism (7) (b) Name the type of reaction taking place in Step 2 and suggest a suitable reagent or combination of reagents. Type of reaction.......................................................................................................... Reagent(s)................................................................................................................... (2) (c) Write an equation for the reaction occurring in Step 3. Name and outline the mechanism for this reaction. Equation Name of mechanism.................................................................................................... Mechanism