metals & nonmetals
advertisement
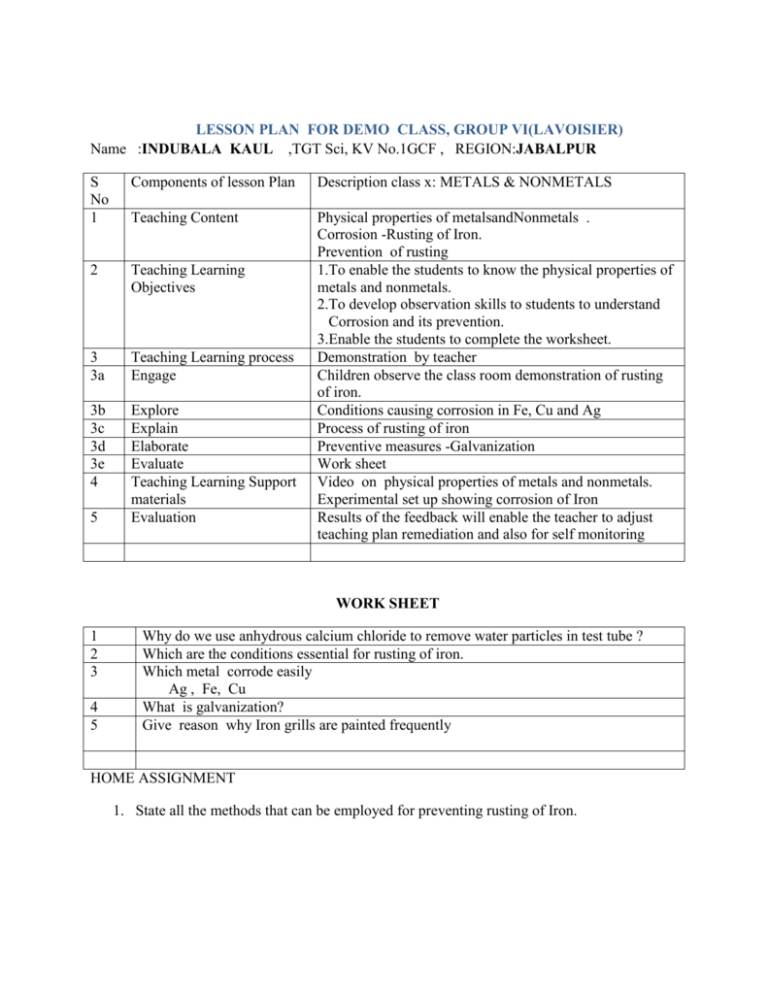
LESSON PLAN FOR DEMO CLASS, GROUP VI(LAVOISIER) Name :INDUBALA KAUL ,TGT Sci, KV No.1GCF , REGION:JABALPUR S No 1 Components of lesson Plan Description class x: METALS & NONMETALS Teaching Content 2 Teaching Learning Objectives 3 3a Teaching Learning process Engage 3b 3c 3d 3e 4 Explore Explain Elaborate Evaluate Teaching Learning Support materials Evaluation Physical properties of metalsandNonmetals . Corrosion -Rusting of Iron. Prevention of rusting 1.To enable the students to know the physical properties of metals and nonmetals. 2.To develop observation skills to students to understand Corrosion and its prevention. 3.Enable the students to complete the worksheet. Demonstration by teacher Children observe the class room demonstration of rusting of iron. Conditions causing corrosion in Fe, Cu and Ag Process of rusting of iron Preventive measures -Galvanization Work sheet Video on physical properties of metals and nonmetals. Experimental set up showing corrosion of Iron Results of the feedback will enable the teacher to adjust teaching plan remediation and also for self monitoring 5 WORK SHEET 1 2 3 4 5 Why do we use anhydrous calcium chloride to remove water particles in test tube ? Which are the conditions essential for rusting of iron. Which metal corrode easily Ag , Fe, Cu What is galvanization? Give reason why Iron grills are painted frequently HOME ASSIGNMENT 1. State all the methods that can be employed for preventing rusting of Iron. LESSON PLAN FOR DEMO CLASS Name :MADHU BHANDARI,TGT Sci, KV No.2,OF DEHU ROAD , REGION: MUMBAI REGION S No 1 Components of lesson Plan Description class x: METALS & NONMETALS Teaching Content Nonmetal oxides 2 Teaching Learning Objectives 3 3a Teaching Learning process Engage 1.to develop observation skill in students to understand that nonmetals react with oxygen to form their oxides that react with water and give acids. 2.To enable the students to know that nonmetal oxides are acidic in nature 3. Enable the students to complete the given worksheet. Demonstration by teacher Class room demonstration--- burning of sulphur in air to form sulphur dioxide Reaction of sulphur dioxide with water toform acidTesting of acid by students Class Response 1.Name the gas formed when sulphur is burnt in air. 2.Give a characteristic property of sulphur dioxide 3.Issulphur dioxide soluble or insoluble in water. 4.What product do you get when nonmetal oxides dissolve in water 3b Explore 3c 3d Explain Elaborate 3e Evaluate 4 Teaching Learning Support materials 5 Evaluation Video presentation showing oxidation of phosphorus then reaction with water to form acids. Giving other examples of nonmetal oxides reacts with water and give acids ( Phosphorous) write balanced chemical equations for the reaction shown. Balance chemical equations of other examples. Role of sulphur dioxide in air pollution and acid rain Activity based Work sheet HOME WORK; Nonmetals are acidic in nature. Write three examples with chemical equations to justify your answer. Sulphur, deflagrating spoon, conical flask with lid, spirit lamp, litmus paper, Manual for formative assessment class x –CBSE VIDEO ON Results of the feedback will enable the teacher to adjust teaching plan remediation and also for self monitoring WORK SHEET 1. Which of the following are acidic oxides :Calcium oxide, Sulphur dioxide, Zinc oxide, Carbon dioxide, Magnesium oxide. 2. What type of oxides are formed when nonmetals combines with oxygen? Give example. 3. How will you test the nature of nonmetallic oxide? 4. Oxide of an element when dissolved in water turns blue litmus to red. State whether the element is a metal or nonmetal? 5. An element form two oxides XO and XO2 . XO is poisonous,XO2 is acidic in nature, soluble in water and extinguishes fire. Identify X. HOME ASSIGNMENT 1. Nonmetals are acidic in nature. Write three examples with equations to justify your answer. LESSON PLAN FOR DEMO CLASS Name :K.VALSALAKUMARI,TGTSci, , KV. KELTRON NAGAR REGION: ERNAKULAM S No 1 Components of lesson Plan Teaching Content Teaching Learning Objectives Description class x: METALS & NONMETLAS 3a Teaching Learning process Engage 3b Explore 3c 3d Explain Elaborate Demonstration of burning of Mg in air to form Magnesium oxide and dissolving in water followed by litmus test s Class room demonstration – students perform experiments to determinethe nature of metal oxides and metal hydroxides Video presentation Through the experiment children write reactions --.> Na, K, Zn and Al Metal oxides with basic and acidic nature Amphoteric oxides Eg; Al2O3 and ZnO Anodising – in case of aluminuim vessels Balanced Chemical equations Chemical formulae of more basic oxides CuO, PbO, FeO, Fe2O3, 3e 4 Evaluate Teaching Learning Support materials EVALUATION 2 3 5 METAL OXIDES 1. To develop observation skill in students and help them to understand that metal react with oxygen to form their oxides 2. To enable the students to know that metal oxides and metal hydroxides are basic in nature 3. Metal oxides react with water to form metal hydroxides 4. To enable the students to understand and write balanced chemical equation 2 Mg + O2 2MgO 5. To enable the students to complete the worksheet Class response VIDEO--https://www.youtube.com/watch?v=d-iqaXSCKUg Formative assessment manual for class X –CBSE Spirit lamp, watch glass, water, litmus Worksheet Results of the feed back of work sheet will be used to diagnose learning difficulties Home Work Metal oxides are basis in nature. Justify the statement with a suitable example WORK SHEET 1 Name two metals that occur in free state in nature 2 3 Sodium oxide is a basic oxide which react with water to form --------- and is called ---------Completethe following reactions a) Magnesium burnt in presence of air b) Calcium and Potassium with water. Why sodium kept immersed in Kerosene Arrange the following metals in the decreasing order of chemical reactivity, placing the most reacting metal first. Cu, Mg, Fe, Na, Ca, Zn What are amphoteric oxides .Give two exmples 4 5 6 HOME WORK 1. Give reason for the following. a) Iron is used in structural engineering b) Lead is used as fuse wire c) Zinc is coated over iron. d) Aluminium is used to reduce metal oxides like Fe2 O3. LESSON PLAN FOR DEMO CLASS Name :POOMANI ,TGT Sci, KV HVF,AVADI , REGION: CHENNAI REGION S No 1 2 Components of lesson Plan Description class x: METALS & NONMETALS Teaching Content Teaching Learning Objectives DISPLACEMENT REACTION 1. To enable students to understand the concept of DISPLACEMENT REACTION 2. To develop observation skill among the children and to help them in constructing knowledge of arranging the apparatus for the reaction (Fe +CuSO4 3 3a 3b 3c Teaching Learning process Engage Explore Explain 3d Elaborate 3e 4 Evaluate Teaching Learning Support materials Evaluation 5 Video presentation,activity More examples of displacement reaction How to identify more reactive and less reactive metals from given displacement reaction Reaction between Copper sulphate and silver Reaction between Silver nitrate and copper ACTIVITY BASED WORK SHEET VIDEO OF DISPLACED REACTION CuSO4 , Fe , Mg, Zn, Fe SO4,Cu Results of the feedback- will enable the teacher to adjust teaching plan , remediation and also for self monitoring ACTIVITY BASED WORKSHEET Name :POOMANI ,TGT Sci, KV HVF,AVADI , REGION: CHENNAI REGION Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows Use the Table above to answer the following questions about metals A, B, C and D. (i) Which is the most reactive metal? (ii) What would you observe if B is added to a solution of Copper(II) sulphate? (iii) Arrange the metals A, B, C and D in the order of decreasing reactivity. LESSON PLAN FOR DEMO CLASS Name :SreejithGopalan ,TGT Sci, KV Kollam ,ERNAKULAM REGION SNo Components of lesson Plan TIME: 20 mts 1 Teaching Content 2 Teaching Learning Objectives 3 3a Teaching Learning process Engage 3b 3c Explore Explain 3d Elaborate 3e 4 Evaluate Teaching Learning Support materials 5 Evaluation Description CLASS ;X METALS & NONMETALS Reactions of Metals with Dilute acids 1.To enable the students to understand how to carry out a hands on practical to learn about the reaction between Acids and Metals 2. To carry out the reaction between dil.HCl and (Mg,Zn,Fe,Al Cu) metals 3. To write the chemical reaction between acids and metals To understand the precautions to be taken during the reaction 4. .Arrange the metals in the increasing order of reaction after observing the reaction TEACHER DEMONSTRATION followed by individual work sheet completion Children perform reaction between dilHCl and (Mg,Zn,Fe,Al Cu) metals in classroom / lab in groups Explores which metal is more reactive Explain the rate at which different metals react with dilute acids by observing the evolution of gas. Elaborates the reaction between sulphuric acid and metals in the similar way Demonstration Experiment based work sheet 1.Experimental set up for carrying out reaction dil .HCl and (Mg,Zn,Fe,Al Cu) metals 2. Formative assessment manual for teachers class X.CBSE 1. Science Activity Based Worksheet 2. HOME Work / Class work. Task: Individual Topic: Reaction of Acids with Metals SCIENCE ACTIVITY BASED QUESTIONNAIRE 1.Which gas is evolved when a dilute acid is reacted with Mg. 2.Which gas is burning with POP sound. 3.Is there any colour for the hydrogen gas liberated during the reaction. 4.Do all metals liberate hydrogen gas in the same rate ( bubbles) 5.Which metal is not liberating hydrogen gas during this reaction 6.Which metal is liberating maximum hydrogen during this reaction 7.How will you arrange the metals in an increasing order of reactivity based on the reaction with dilute acid 8. Which set up recorded the maximum temperature? 9. Which set up recorded minimum temperature 10. What name do you give for chemical reaction which give out heat? (10 x 1=10) Complete the following Student worksheet Read the question and answer the following 1 A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used? 2 Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below. (a) What will be the action of gas on (i) dry litmus paper? (ii) moist litmus paper? (b) Write a balanced chemical equation for the reaction taking place.








