the effects of ph on enzymes
advertisement

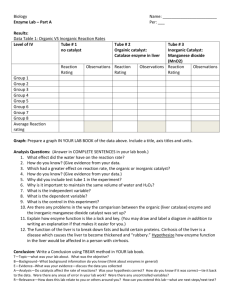
THE EFFECTS OF pH ON ENZYMES Zachary S. Woolsey Introduction Right now in Biology we are studying the meaning of words that many of us have heard at some point in our lives. Even while watching television it would not be surprising to hear words like enzymes, pH and catalysts. However, we might not know the exact meaning or how they even work together. According to our lab manual, “Living cells use organic catalysts, or enzymes to speed up chemical reactions... Cellular chemical reactions would not occur at rates rapid enough to support life without the aid of enzymes.” (Concepts of Biology Laboratory Manual, Boise State University 2008) Therefore, enzymes are an essential part of living organisms. Enzymes are proteins that break down chemical bonds. “Enzymes act by stressing the chemical bonds of reactants, which decreases the amount of activation energy required to start the reaction.” (Concepts of Biology Laboratory Manual, Boise State University 2008) The pH, or potential Hydrogen, is a measurement of the concentration of hydrogen ions in a given solution. Every enzyme has an internal pH. The pH of a separate solution is an environmental factor that can disrupt an enzyme’s activity and structure. pH is also used to measure the acidity or alkalinity of a solution. The pH has a numeric scale of 1 to 14; 1 being most acidic, 7 being neutral and 14 being most alkaline or basic (Belk and Borden 2007). In our study we will determine the effects of pH on enzyme activity in catalase beef liver cells. We will add various solutions with different pHs to liver cells. We will then measure the chemical activity this reaction produces by the thickness of the foam layer. A thick foam layer will demonstrate a high enzyme activity. I hypothesize that a high pH level will result in high levels of enzyme activity. Materials and Methods First, place four drops of liver into 8 10 ml test tubes. Label each one with one of the following pH levels: 2,4,6,8,10,12,14. An additional beaker will be marked “water” (this will be used as our control). Second, add 1 full dropper of water (H2O) to each of the liver samples in each tube. Third, using a dropper, add a full drop of the solutions containing pH levels of 2,4,6,8,10,12, and 14 to their corresponding beakers. Forth, add one dropper of Hydrogen Peroxide (our reactant) to each of the 8 test tubes. Fifth, mark each of the beakers at the bottom and top of each foam layer. Sixth, with the ruler provided, measure each foam layer in millimeters. Finally, record your results. Results Table 1. Results Data Sheet Data Sheet Tube Water pH 2 pH 4 pH 6 pH 8 pH 10 pH 12 pH 14 Thickness of Foam Layer (mm) – Catalase Activity 0 mm 28 mm 24 mm 20 mm 28 mm 38 mm 3 mm 2 mm Figure 1. Results Bar Graph 40 35 30 25 20 15 10 5 0 pH 14 pH 12 pH 10 pH 8 pH 6 pH 4 pH 2 Foam Layer Water Foam Layer Thickness (mm) Results Bar Graph Catalase Activity Table 1 and Figure 1 illustrate the enzyme activity that occurred with each pH level tube tested. Catalase activity will show a formation of foam in a tube. The figure and table show that the least amount of catalase activity recorded was found in the test tube labeled “water” (no activity). The highest amount of catalase activity was found in test tube “pH 10” with a foam thickness of 38 mm. Discussion Based on the results of our experiment, we can see that the reaction from the eight beakers varied in foam layer thickness. Because there was not a positive correlation of foam thickness in relationship to the increase (or decrease) in pH levels, I must reject my hypothesis that the higher the pH level the thicker the foam layer. Catalase activity was so inconsistent that it was difficult for me to even find any sort of patterned behavior. For example, with the pH 10 beaker I found the most catalase activity. The pH 12 beaker was tested next and showed one of the least amounts of catalase activity. We must consider that there could have been errors in the experiment to produce a misleading result. It is very possible that there was not enough of the liver enzyme present in some of the beakers, resulting in a low production of foam. The pH may have acted as a buffer by denaturing and changing the internal pH of the enzyme itself. This would have caused the enzyme to stop functioning. Environmental factors may have contributed to enzyme deterioration or poor enzyme productivity. A couple of examples of possible environmental factors leading to faulty results are as follows: How long the liver enzyme was detached from its previously living organism. Temperatures the liver enzyme may have been exposed to prior to the experiment (either too hot or too cold). Suggestions for improvement may include: Extracting a live enzyme from a living organism and directly transferring it to a beaker for testing. Preparing the lab environment temperature for optimum enzyme activity. Testing the pH of the enzyme itself before testing it with other pH solutions. By testing the pH levels of enzymes, we might be able to better understand the enzyme activity occurring in human beings. As we increase our knowledge of catalase reactions to pH levels, we may gain insight into such diseases as scorosis of the liver, cancers, or maybe even find ways to improve overall health and digestion. Sources Cited Boise State University Department of Biological Sciences. Edited by Judy Lonsdale, MS. 2008. Concepts of Biology Laboratory Manual. Kendall/Hunt Publishing Company. Belk and Borden. 2007. Biology: Science for Life with Physiology. Prentice Hall.