SOLUBILITY EQUILIBRIUM
advertisement

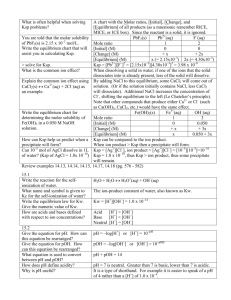
SOLUBILITY EQUILIBRIUM Terminology you should already be familiar with: Unsaturated Saturated Supersaturated Strong Electrolytes: are completely soluble in water. Weak electrolytes: are slightly soluble in water (slightly dissolve and dissociate in water) Example: Silver acetate is a weak electrolyte. As the ion concentration increases, the reverse reaction occurs (precipitation) until equilibrium is reached. At the equilibrium, the solid has dissolved as much as it can and is at it’s maximum solubility (S in mol/L). Equilibrium Law for the above reaction can be expressed as: Where Ksp: stands for the solubility product applies to a saturated solution. A(s) B(aq) @ eq’m rdissolution = rprecipitation There are two ways of establishing equilibrium between excess solute and an aqueous solution. 1. Dissolving excess salt into a solution until a saturated solution is reached. 2. Mixing two aqueous salt solutions together to precipitate the product out. Example: Calculate the Ksp of magnesium fluoride at 25C, given the solubility of 0.00172g/100mL. Write balanced chemical equation: Equilibrium Law: Use given information to set-up an ICE table: Solve for Ksp: Will a precipitate form? Recall: Q is the reaction quotient used to determine if a particular reaction is at equilibrium or not. When considering solubility equilibria, the Qsp (the ion product) of a reaction can be calculated using any values that may or may not be at equilibrium. The significance of calculating the Qsp is that it enables one to depict whether a precipitate will form of the given ion concentration. Compare Qsp to Ksp Qsp < Ksp Qsp = Ksp Qsp > Ksp No ppt, solution unsaturated No ppt, solution is saturated PPT, solution is supersaturated Steps to solving problems: 1. 2. 3. 4. 5. 6. 7. Write the net ionic equation. find moles of each ion. determine the total volume. Use new values (step 2 and 3) to determine new concentration. Find the Qsp Compare Qsp to Ksp If Qsp > Ksp, therefore a ppt will form. Example: if 100mL of 0.100 M CaCl2(aq) and 100mL of 0.04M Na2SO4(aq) are mixed at 20C, determine whether a precipitate will form. The Ksp is 3.6 x 10-5. The Common Ion Effect Adding an ion will shift the equilibrium, if the ion/compound reacts with the ions already present in solution. For example, magnesium fluoride is a slightly soluble salt, what direction will the equilibrium shift to, if a solution of sodium fluoride is added to the solution? Note: If the slightly soluble salt (MgF2) is dissolving in a solution of NaF less of the salt will dissolve due to the presence of the common ion (F-). Example: What is the solubility of a Magnesium fluoride into a solution of a 0.04M NaF solution. (note: assumptions can be made, as long as they are valid).