d. Sparklettes
advertisement
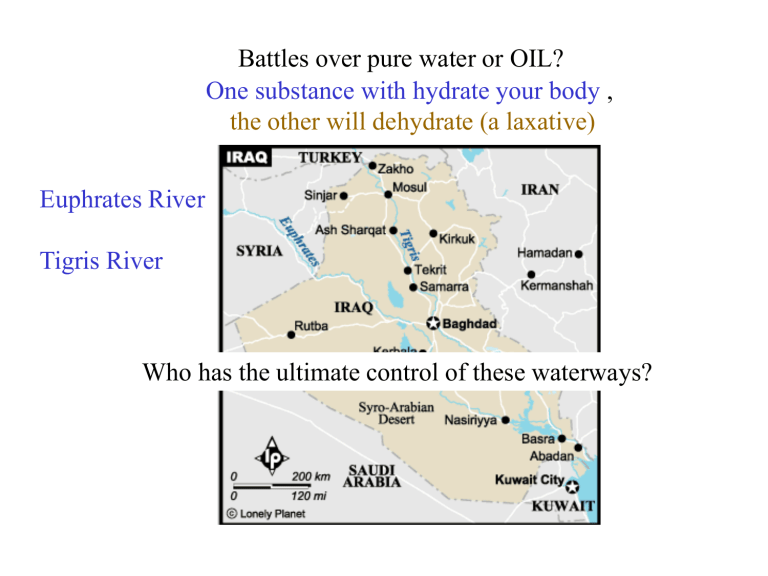
Battles over pure water or OIL? One substance with hydrate your body , the other will dehydrate (a laxative) Euphrates River Tigris River Who has the ultimate control of these waterways? Sparklettes Water Dr. Gergens - SD Mesa College The Crystal-Fresh® Drinking Water ingredient label says the following: “Drawn from our deep protected wells in Santa Ana, CA. Purified using our Crystal-Fresh process, including filtration, ozonation, reverse osmosis, and/or dionization. Contains purified water and specially selected minerals in nutritionally insignificant amounts for great taste (sodium bicarbonate, magnesium chloride, calcium chloride and sodium sulfate). Sparklettes Water Dr. Gergens - SD Mesa College Lets learn to write the correct formulas for these substances (sodium bicarbonate, magnesium chloride, calcium chloride and sodium sulfate) that Sparkletts ® adds to it’s purified water In “nutritionally insignificant amounts for great taste.” Sparklettes Wate r Nomen cla ture Exe rcis e: "Nu triti onal ly ins igni ficant amou nts of these co mpou nds adde d fo r goo d ta ste." Dr. Gerge ns - SD Me sa Col lege + Supplemental packet page 76 – 1. Write th e na me e ach ca tion and each a nion (e.g ., Na is sod ium ion; Cl is ch lorid e io n) 2. Say an d write the nam e of the ion ic salt co mpou nd b y com bini ng e ach ca tion with each an ion in the table (e.g ., s odiu m ch lorid e) 3. Compl ete the tabl e by writing in the i onic sa lt comp ound form ula in e ach ce ll o f th e ta ble (e.g., NaCl). 4. Whe n writi ng a form ula a catio n an d an ion must co mbin e in an appro priate ration to b alan ce cha rge; see exampl es o n back. anions (name these ions) cations (name these ions) Na + sodium ion Mg 2+ magnesium ion Ca 2+ calcium ion – Cl chloride ion NaCl sodium chloride MgCl2 magnesium chloride CaCl2 calcium chloride SO4 2- sulfate ion Na2SO4 sodium sulfate MgSO4 HCO3 – hydrogen carbonate ion NaHCO3 sodium hydrogen carbonate Mg (HCO3) 2 magnesium sulfate magnesium hydrogen carbonate CaSO4 calcium sulfate Ca(HCO3) 2 calcium hydrogen carbonate 5. Predi ct the trans itio n me tal cation cha rge for iro n, Fe, i n th e io nic sal t Fe 2 (SO4 ) 3 , a nd p lace it in the cation box be low. 6. Give a name for Fe 2 (SO4 ) 3 . Since tra nsition meta ls can va riabl e charge , you m ust some how i ndicate metal cati on charg e in its name . 3+ – – 7. Write ad diti onal form ulas for the cation Fe co mbin ed with th e an ions Cl an d HCO3 an d gi ve the ir compo und name s. cation iron (III) ion FeCl3 iron (III) chloride Fe 2 (SO 4 ) 3 iron (III) sulfate Fe(HCO3) 3 iron (III) hydrogen carbonate Acids . In g enera l, a sub stan ce that has an 'H' listed first in i ts formul a is refe rred to as an acid . Na me the acid b ut p lace a prefi x in its name di = 2, tri = 3, tetra = 4, pen ta = 5, hexa = 6, hepta = 7, o cta = 8 , no na = 9, deca = 10 to in dicate the numb er of hydrog ens in the formul a. anions cations H+ hydrogen ion give a common name and use for each acid – Cl HCl SO4 2- H2SO4 HCO3 – H2CO3 hydrogen chloride hydrogen chloride dihydrogen carbonate hydrochloric acid sulfuric acid carbonic acid stomach acid car battery acid carbonated water Calculation of Oxidation State Fe2(SO4) 3 We are looking for balance in charge 2+ 2- cation anion 2+ cation 1+ 1+ cation 11- = 0 Fe = Fe = (SO4)2- (SO4)2- 0 anion 2- 3+ 3+ 0 (SO4)2- anion iron (III) ion total charge of positive 6 sulfate ion total charge of negative 6 What must be the charge over the two iron ions to balance the sulfate ion charges? ratio of 2 Fe 3+ : 3 (SO4) 2For Charge Balance: two iron(III) ions for every three sulfate ions tutorial http://homework.sdmesa.edu/dgergens/chem100/nomenclature/naming_practice.htm


