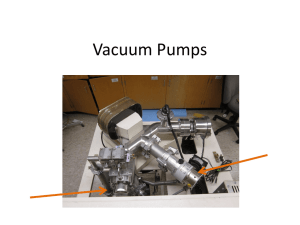
Anatomy of an IRMS Lecture 2 – Vacuum Systems Reading: see
advertisement

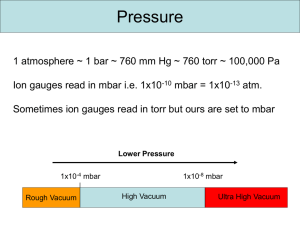
Lecture 2 – Vacuum Systems Anatomy of an IRMS Reading: see www.vacuumlab.com/articles 1) Introduction – Start by looking at the molecular basis of pressure and vacuum a) Units i) Atmosphere (atm). Very convenient, not commonly used. ii) SI unit is Pascals (Pa), defined as 1 N/m2 . 1 atm = 101,325 Pa. Very inconvenient for typical calculations, but still should be used in publications. iii) Torr – most common in US; 1atm = 760 Torr iv) Bar (mbar) – most common in Europe. 1 atm = 1.0135 bar. v) See conversion scale to go between them. To rough approximation, 1mbar = 1 Torr, 1 mTorr = 10-3 mbar. b) Vacuum Regimes Regime Pressure (Torr) Pressure (mbar) Molecules/mL Mean free path Low vacuum 25 33 1018 2 um 13 medium 1e-3 1.33 10 10 cm High 1e-9 1.33e-6 107 100 km Ultra high (UHV) 1e-12 1.3e-9 104 100,000 km Interplanet space 1e-15 10 c) Flow regimes i) Molecular flow can be categorized in two regimes by comparing mean-free path to characteristic distances of system (ie, tube diameter); Knudsen number ii) Mean free path a function of density and size: mfp = (sqrt(2)*pi*n*d2)-1, n= molecules/cm3, d = molecular diameter (cm); thus smaller atoms (He) have a longer mean free path than larger ones (N2, Ar) iii) Viscous flow – dominated by interactions between molecules. Pressure-driven flow. Behaves like water in pipes. Does not fractionate isotopes. iv) Molecular flow – dominated by collisions with walls. Diffusion-like flow, rate dependent on mean-free path and probability that a gas molecule flies into pump orifice. Does fractionate isotopes, because velocity varies with mass. v) Transition between viscous and molecular flow depends on geometry of physical system. Smaller diameters will experience viscous flow at much lower pressures. (see graphic). Becomes very important in talking about dual-inlet systems. d) Pump-down i) At low vacuum, flow is dominated by viscous flow, simply need to minimize friction losses. ii) At high vacuum, system is in molecular flow. Rate of pumping down is governed by the mean-free path of molecules out of the system. Can be formalized as the conductance of the system, which is the inverse of resistance. iii) Math. Can be described in analogous way to electricity; recall V = IR (1) V is equivalent to pressure (P; units Torr) (2) I is equivalent to mass flow rates (Q; units Torr-L/sec) (3) Conductance then has units of L/sec; tubes and orifices have a conductance, but so do pumps; in pumps this is often called the pumping speed (S), where it reflects a volumetric 1 Lecture 2 – Vacuum Systems Anatomy of an IRMS flow rate. Note that pumping speed of a physical pump (turbos) depends on size of molecule, and is slower for smaller atoms; (a) The speed of a pump is quoted as the volumetric flow per unit time; will be ~constant over some pressure range, but realize that this represent decreasing mass flow rate (4) Combining these we get P = Q/S, usually rearranged to give Q = SP (a) At fixed mass flow rate (determined by inputs), higher pumping speed or lower conductance gives lower pressure (b) At fixed pressure, higher pumping speed gives higher flow rate (5) When we have multiple components in series (tubes then pump), calculate the effective conductance just as we would for resistance (Reff = R1 + R2 +…; 1/Seff = 1/S1 + 1/S2+…) iv) These calculations are often very useful in diagnosing vacuum systems. If mass flow rate and pressure are known, pumping speed can be calculated. If pumping speed and pressure differential are known, a limiting conductance can be calculated. In a closed ion source, if conductance is known then pressure inside ion volume can be calculated. e) Pump-down curves i) Volume zone (volume of system); only matters during initial pumpdown, usually cleared in a matter of seconds to minutes; pressure reaches 1 mbar quickly ii) Below that, flow is dominated by (1) Desorption from walls, mainly water; in this regime pumpdown time is dominated by humidity/exposure time and surface area, not volume; called the “drydown zone” of the pumping curve; this is why we vent instruments with dry N2 instead of to air (a) Rate of desorption depends on supplying enough energy to drive molecules into the gas phase; water is adsorbed in multiple layers, with layers closer to the surface more strongly bound, so rate of desorption slows with time; increasing temperature increases ambient energy and speeds the rate of desorption (b) Note that most water molecules desorb, hit another surface, and then many resorb (sticking coefficient is about 1%); thus the geometry of the system plays important role in rate of desorption (c) Common ways to spead up water removal are baking (200-250C) and UV photons (2) Leaks through seals (o-rings or gaskets); assuming no outright leaks, rate is generally determined by permeability of the seal; polymer o-rings have much higher rates than metal gaskets; Viton is better than buna iii) Turbo pumps can typically reach 10-6 mbar via baking out; below that, pressure is mostly H2 which is not efficiently pumped, thus need to use ion pumps to get better vacuum f) Designing vacuum systems. i) Sizing tubing. Generally want wider diameters and shorter length. Diminishing returns as surface area increases. ii) Straight flow paths. iii) Smooth surfaces adsorb less water than rough ones (surface area) iv) Pump speed. Higher is better, but costs increase exponentially. Need to consider material being pumped; air is much easier to pump than He v) Minimize number of seals to reduce permeation; tradeoff between lower leak rates (metal) versus convenience (polymer) vi) Virtual leaks. Air trapped at end of a screw has very long free path, will gradually leak out and take forever to pump down. Special design to provide eliminate trapped air spaces vii) Cleanliness. Oil and fingerprints degas very slowly (low vapor pressure), and at high pressure the equivalent volume of a small mass is very large (1) Calculation: 1mg of a substance with 100g/mol would have a volume of 22.4 E-5L at STP. At high vacuum (1E-6 mbar, or 1E-9 atm) this becomes 22.4 E4 L or 224,000 liters. 2) Pressure gauges 2 Lecture 2 – Vacuum Systems Anatomy of an IRMS a) Review gauge pressure versus absolute pressure. Gauge pressure depends on local atmospheric pressure, really only useful for high-P measurements. b) Mechanical – work by measuring displacement or strain under pressure; useful for ~1 Torr to high pressure i) Manometer- measure the height of liquid displaced (water or mecury) (1) McLeod gauge; compresses gas into a liquid manometer; very accurate, down to 10-6 torr, but inconvenient; often used for calibration of other gauges ii) Anaeroid – metal pressure plate pushes against a spring and displacement is measured iii) Bourdon tube – a flattened tube tends to become more circular as pressure differential increases; when tube is bent into C shape, this causes it to straighten out at pressure drops; can be surprisingly sensitive iv) Diaphragm gauge – measures deflection of a flexible membrane. Often implemented as a ‘capacitance manometer”, in which system measures capacitance of the membrane moving relative to a fixed plate of known capacitance. Baratron gauge is one example. Useful down to 10-2 Torr (mbar) c) Thermal conductivity (useful range: 10-3 to 10 Torr) i) Basic principle is that the rate of heat loss of a wire in vacuum depends on number of molecular collisions, which depend on density of molecules. Drawback of all these is that they depend on heat capacity of gas, must be calibrated for specific gases. ii) Thermocouple gauge; constant current applied to wire, separate thermocouple junction heated by conduction measures temperature of the wire. This is a 2-wire system. iii) Pirani gauge. Uses a single platinum wire, applying constant current and measuring change in voltage as the resistance of the wire changes with temperature. d) Ionization gauges (useful range: 10-10 to 10-3 Torr) i) Bombard gas with electrons, which ionizes gas and causes a resulting current. ii) Hot cathode design – uses a heated filament to boil off electrons (thermionic emission) iii) Cold cathode design (Penning gauge, magnetron) – uses high voltage (4kV) to induce current flow; more sensitive and less maintenance, but more difficult to use 3





![Pirani Vacuum Gauge [GP-G Series]](http://s2.studylib.net/store/data/018216349_1-c59e249bdf583a004cb04f73e8f6a27f-300x300.png)