1_1_Vaccum_fundamentals
advertisement
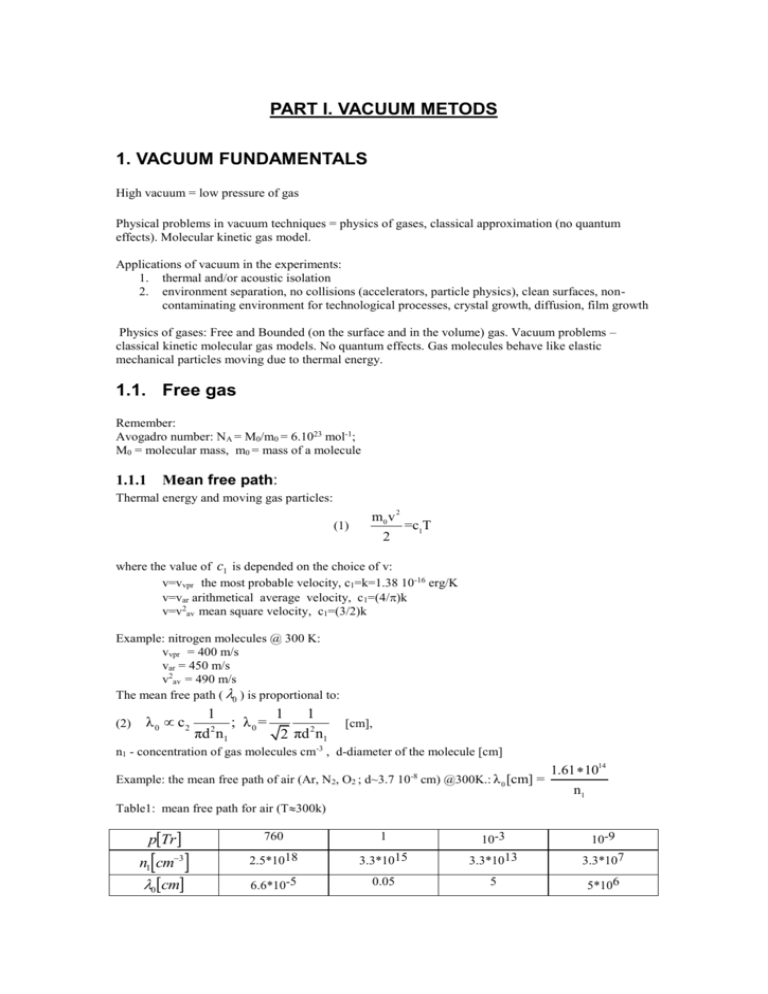
PART I. VACUUM METODS 1. VACUUM FUNDAMENTALS High vacuum = low pressure of gas Physical problems in vacuum techniques = physics of gases, classical approximation (no quantum effects). Molecular kinetic gas model. Applications of vacuum in the experiments: 1. thermal and/or acoustic isolation 2. environment separation, no collisions (accelerators, particle physics), clean surfaces, noncontaminating environment for technological processes, crystal growth, diffusion, film growth Physics of gases: Free and Bounded (on the surface and in the volume) gas. Vacuum problems – classical kinetic molecular gas models. No quantum effects. Gas molecules behave like elastic mechanical particles moving due to thermal energy. 1.1. Free gas Remember: Avogadro number: NA = M0/m0 = 6.1023 mol-1; M0 = molecular mass, m0 = mass of a molecule 1.1.1 Mean free path: Thermal energy and moving gas particles: (1) m0 v 2 2 =c1T where the value of c1 is depended on the choice of v: v=vvpr the most probable velocity, c1=k=1.38 10-16 erg/K v=var arithmetical average velocity, c1=(4/k v=v2av mean square velocity, c1=(3/2)k Example: nitrogen molecules @ 300 K: vvpr = 400 m/s var = 450 m/s v2av = 490 m/s The mean free path ( 0 ) is proportional to: (2) λ 0 c2 1 1 1 ; λ0 = 2 2 πd n1 2 πd n1 [cm], n1 - concentration of gas molecules cm-3 , d-diameter of the molecule [cm] Example: the mean free path of air (Ar, N2, O2 ; d~3.7 10-8 cm) @300K.: λ 0 [cm] = 1.61 1014 n1 Table1: mean free path for air (T300k) pTr n1cm3 0cm 760 1 3.3*1015 10-3 3.3*1013 2.5*1018 6.6*10-5 10-9 3.3*107 0.05 5 5*106 1.1.3. Pressure. The frequency of collision of gas molecules with the wall f1 ' is proportional to concentration and velocity: (3) f1 ~ c3n1v when f1 [s-1cm-2], n1 [cm-3], v=vav then : c3 = 0.25 The momentum transfer at an elastic collision with the wall: m0v-(-m0v)=2m0v. The force on a unit surface (pressure) pressure is: (4) p = 2m0 vc3n1v= 2c3n1m0 v 2 c4 n1m0 v 2 where c4 is a proportionality constant that depends on the choice of pressure units and the definition of the velocity. 1.1.3.1. Pressure units Torr, 1Tr = 1 mm Hg Physical atmosphere, normal atmosphere, 1 Atm = 760 Torr Technical atmosphere, 1 At = 1Kg/cm2 Pascal; 1 Pa = 1 N/m2 = 1.02 *10-5 At =0.99 Atm=0.75*10-2 Torr 1 bar = 103 mbar = 7.5*102 Torr =105 Pa = 1 Hectopascal 1 mbar = 0.75 Torr 1 PSI = 1pound/inch2 conversion table: to be inserted 1.1.3.2. Relation between pressure, concentration, and temperature. m0 v 2 Express m0v as a function of T . From (4) we have p=c 4 n1m 0 v and since =c1T we get 2 (5) p=2c1c4 n1T=c5n1T 2 2 when T [K], p [Tr], n1 [cm-3] then: c5 =10-5 and: p Tr 10-19 n1T (6) From (5) we can get for the concentration n1 : (7) n1 = p c 5T For Air (d~3.7 10-8 cm) p r] . At room temperature p [Tr]. N.B. In a closed container a temperature change will not change since simultaneously the pressure is changing. 1.1.4. Properties of the Air Table 2: Air - components N2 O2 Ar CO2 Ne He % vol.’ 78 20.95 0.93 0.03 0.002 52*10-4 % weight 75.5 23.14 1.25 0.05 0.001 72*10-5 pTr 590 160 7 0.25 0.014 4*10-3 H2 5*10-5 5*10-6 4*10-4 Air pressure changes with altitude. Table 3: atmospheric pressure vs. altitude Alt.[km] 0 14 30 760 100 10 pTr 47 1 78 10-2 100 10-4 200 10-6 480 10-8 In between the sea level and about 14 km pressure changes almost linearly with the altitude. For calibration of pressure meters an absolute meter is needed as a reference. 1.1.5. Vacuum ranges Vacuum classes corresponding pressure ranges Forvacuum (technical) p>10 -3 Low vacuum (LV) 10-3>p>10-6 High vacuum (HV) 10-6>p>10-9 Ultra high vacuum (UHV) 10-9>p>10-12 Extremely high vacuum p<10 -12 “Clean” and “dirty” vacuum: possible contaminations water, oil, specific contaminations, helium, noble gases, hydrogen. 1.1.6. Amount of gas and the state equation of gas The product Q=pV is referred to as an the amount of gas in pV units [Tr*l], [Pa*m3], etc. Boyle- Mariotte`s law (B-M law): (8) ρ=n1m0 , n1 = ρ= m V m 1 , m0 mass of a gas molecule m0 V from (5) we have : (9) p=c5n1T=c5 m 1 T, m0 V and we obtain the B-M law for the amount of gas (Q): (10) Introducing the universal gas constant Q=pV=c5 R=c5 N A m m T=c5 N A T m0 M0 QR (11) m T. M0 Expressing m in [g], M0 in [g/mol], T in [K], p in [Tr], and V in [l] R=62.37. In other units R=8.32 104 J/(mol *K), etc. 1.1.7. Flow of gas, intensity and velocity of flow Intensity of flow is defined as an amout of gas (Q) flowing in a unit time by a cross-section perpendicular to the direction of the flow: I (12) Using B-M law we have: dQ dt I c5 (13) T dm dn c5 T m 0 dt dt were n=nm0 stands for the number of gas particles. The velocity of flow S is defined as: S= dV dt p Units of S; [m3/h], [l/s]. etc. When S<0 we have the pumping effect When S>0 we have leak or degassing effect. I= (14) From (14) we get the relation (16) dQ d(pV) dV p= p =p dt dt dt I S= p p 1.1.8. Intensity and velocity of pumping by an ideal pump Consider an opening leading to an ideal vacuum (ideal pump). The factor which determines the escape intensity (pumping intensity) is the number of collusion with the opening per unit time, Using 3 and 13 we get for the intensity: (17) I=c5T dn =c5Tf1=c3 2c1 dt T p m0 f’- number of gas particles hitting a unit surface in a unit time, p – pressure in the pumped volume. Using (16) we obtain the velocity of flow through an opening of an area A: (18) SA =c3 2c1T A m0 Observe that the velocity of flow (pumping speed) decreases with increasing mass of gas molecules, temperature and evidently is proportional to the surface of the opening. Example: @300 K SA for Air (m0~ 5*10-25 g) = 11.7 A, while for H2 (m0~ 3.4*10-24 g ) SA 45A . 1.1.9. Change of pressure at a constant velocity of flow. Consider a container of a constant volume V that is connected to a pump with a constant pumping speed S. How pressure in the container changes in time? From (13 ) I c5 T dn n , and, on the other hand I=pS=c5n1TS=c5 TS , therefore dt V c5 T dn n dn S c5 TS dt dt V n V and since number of particles is proportional to the pressure we can write S - t dp S = dt p t =p0e V p V (19) Thus pressure exponentially drops to zero with elapsing time. In the experimental reality we do not have an ideal vacuum. There is some finite limiting minimum pressure p available with a particular pump: (20) p t =p +p0e S - t V P P0 P. t Consider an inverse process. We pump down, and after a time t stop pumping: p desorbtion Pumpd-down End of pumping This can be employed to check for leaks in the system. 1.1.10 Flow of gas through tubes There are three distinct regimes of flow in tubes. t 1.1.10.1. Molecular flow In this regime the free path of the gas is longer than the diameter of the tube. Each particule behaves individually. There are almost no collisions between the molecules. Thy collide mostly with the walls of the tube. After a collision the particle velocity direction is random and therefore part of the molecules is “back-scattered” towards the input. In this case the effective mean free path is λ= D , where D is the diameter of the tube. 3 For air we have molecular flow for p/D<2*10-2 mbar/cm 1.1.10.2. Laminar flow Most of the collisions are between the molecules. The flow has a velocity distribution with the maximum in the center of a tube. λ< D 100 For air p/D>0.7mbar/cm . The flow is laminar as long as Q / D 150 mbar l s cm Laminar flow is characterized by low Reynolds number. 1.1.10.3. Turbulent flow Q / D 400 mbar l s cm 1.1.11. Resistance for gas flow Speed of pumping of an ideal pump (opening to an ideal vacuum) is given by (18). However, when the opening is connected to the pumped volume by a tube the pumping speed is reduced due to a resistance to the gas flow in the tube. This is due to a friction resulting from collision of molecules with the walls and between themselves. Molecular conditions L S’ S p=0 The longer and the narrower is the tube, more collisions, the higher is the resistance for the flow W. Tube resistance W follows from the difference in flow velocities 1 1 . S` S Note that only for when L 0 S' S and W=0. W Usually one defines the conductivity of a tube, G 1 , units of G: [l/s] W For a cylindrical tube with L>5D one can use the following approximation: G[l / s] 5 10 12 T D3 T D3 (D and L in cm) 3.8 m0 L M0 L For air @300K D3 G[l/s] 12.2 [cm 2 ] . L Example: A circular tube with D=1cm and L=10cm has a conductivity of G=1.22l/s for Air at 300K. If this tube is connected to the opening of the same diameter leading to the ideal vacuum the speed of pumping of the opening will be S=9l/s. The speed of pumping at the tube inlet will be however: 1 1 1 1 = + 0.8+ = 0.9 [l/s] S` =1.1 [l/s] ` S G S 9 N.B. Even such a short tube reduces the speed of pumping from 9 l/s to 1.1 l/s! Proper design of vacuum systems and pumps: short and wide tubes (large apertures create however other problems). For an opening: the velocity of escape through an opening = the aperture (hole) conductivity G 0=1/W0 Since the impedance of a hole is not zero, in general, the resistance of a tube is: Whole W Whole Wtube , thus even for L 0 , W 0, and W 1.1.11.1. Connecting tubes Similarly to electrical circuits: W Wi i G Gi i 1 for a serial connection, and G 1 for a parallel connection. W 1.1.11.2 Pumping speed at the other end of a tube. S- pumping speed, G- tube conductivity and the pumping speed at the other end of a tube S' S , 1 S / G Therefore, for G>>S the pumping speed S=S` and is not reduced by the tube. However, when G<<S then S`=G and the pumping speed is limited not by the pump used but by the piping. Always adjust pump to the tube and/or the tube to the pump. 1.2. Surface bounded gas 1.2.1 Desorption p() Gas molecule desorbing molecules. departing from the surface. Angular distribution of velocities of The time of permanence on the surface: p 0 exp( Wdes / Ts ) is the time it takes for a molecule to detach itself from the surface (desorption). Ts is the temperature of the surface, Wdes and 0 depends on the molecule and the surface. For higher surface temperature the time for detachment decreases. In free and clean surface the first layer of gas molecules is bonded by metallic bonds (exchanging of electrons). Next layers are attached by atomic (covalent) bonds (deformation of electrons orbitals). Noble gases – van der Waals bonds – weaker bonds “physical bonds” Bonding energies ( Wdes ) in kcal/mole for a clean W surface He 2 Kr 4.5 H 74 CO2 122 O 147 O2 194 N2 85 N 155 Chemical bond Physical bond 1.2.2. Absorption Permanent permanence of the gas molecule on the surface. In the act of absorbtion a latent heat of absorbtion is released and the gas particle remains bounded. For this reason it is hard to absorb noble gases – low energy of bonding. 1.2.2.1. Attachment coefficient and the degree of covering the surface The frequency at which gas molecules are hitting the surface is f '1 c 3 2c 1 c5 p m 0T , but only a fraction of those hits are effective (in a sense of attachment to the surface), f ' eff f ' 1 , where 1 is the attachment coefficient. The degree of covering is N / N 1 , where N is the number of particles located on the surface, N 1 is the number of the available sites on the surface. With increasing , decreases. The absorption speed depends on p, T, and the surface state through the coefficient . The time of total surface covering c m 0T N1 c N 5 1 f '1 c 3 2c1 For air @ 300K and for =0.5, r] ~ 1 second r] ~ 1 hour r] ~ 1 month p τc [s]= 10-6 , meaning that p[Tr] 1.2.3 Surface and vacuum Good vacuum needs clean surfaces – mechanical, chemical, ion, and thermal cleaning Surface analysis – possible only in the UHV vacuum conditions The intensity and velocity of adsorption / desorption from the surface A exposed to an ideal vacuum I A c 5 Tf ' eff A c 3 2c1 The velocity of adsorption/desorption m0 TpA S A c 3 2c 1 m0 TA . For air at 300K, SA [l/s] 12γA . The effective absorption frequency was given in the previous section. For the desorption frequency N N c6 exp( Wdes / Ts ) , where N is the number of molecules on the surface. p 0 In equilibrium f ' abs f ' des , and we can solve for N: f ' des c 6 N c1 c 3 0 p exp( Wdes / Ts ) c5 c 6 m 0 T 1.2.3. Evaporation and condensation Equilibrium vapour pressure above a surface of a material is p p p p 0 exp( Wp / Ts ) insert graph of the vapour pressure vs temperature for various materials. Insert table of melting and boiling temperatures of various elements. 1.2.4. Materials for vacuum systems MOOV 1H13N9T and 1H13 1.3. Gas in the Solid State 1.3.1. Solubility of gas in metal and glass Noble gases: Henry`s low: Q r r1 p , where Q r is the quantity of gas in pV units referred to 760 Tr and 1cm3. Q r r2 p Notice that solubility coefficients r1 and r 2 have different units. These coefficients are temperature Diatomic gases: Sieverst’s low : dependent. In general increase with temperature, stronger for metals than for glass. For glasses: r1 ~0.005 – 0.01 in the temperature range (20-400o C) For metals: r2 ~ 0.001- 1 in the temperature range (200-1000o C) The dissolved gas moves in solids by diffusion: W D D 0 exp d . Ts 1.3.2. Penetration through the walls The amounts of gas that penetrates through 1cm2 along the distance L in 1s is: r D p 2 p1 Q 1 L r D p 2 p1 Q 2 L The permeability coefficient is defined as: W rD 0 exp d , where 0 rD0 , Ts insert graph and 2 tables General requirement for vacuum materials: low permeability!