Pressures and Pumps
advertisement
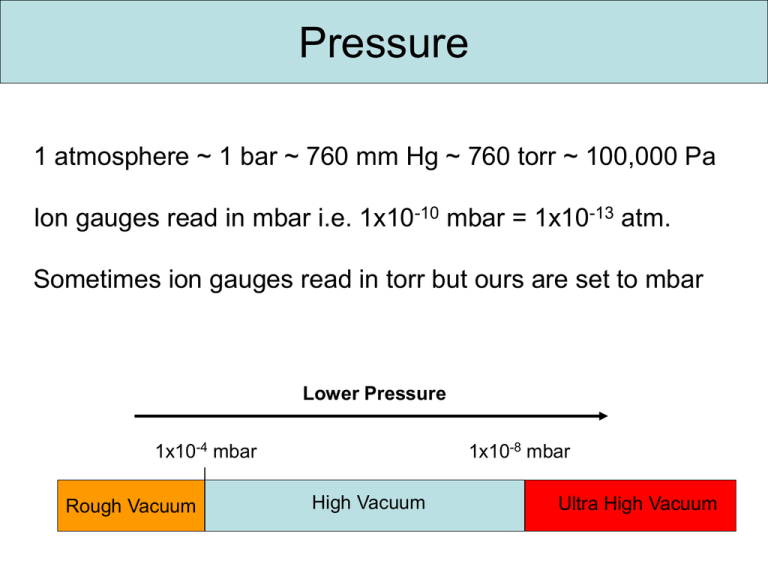
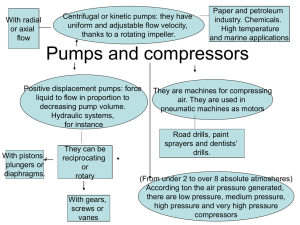
Pressure 1 atmosphere ~ 1 bar ~ 760 mm Hg ~ 760 torr ~ 100,000 Pa Ion gauges read in mbar i.e. 1x10-10 mbar = 1x10-13 atm. Sometimes ion gauges read in torr but ours are set to mbar Lower Pressure 1x10-4 mbar Rough Vacuum 1x10-8 mbar High Vacuum Ultra High Vacuum Pressure Pressure Range Pressure/ mbar Low Vacuum 103-100 Medium Vacuum 100-10-3 High Vacuum 10-3-10-7 Ultra High Vacuum 10-7-10-12 Extreme Ultra High Vacuum Pressure (mbar) Molecular density at 298 K Molecular mean free path λ N2 at 295 K Monolayer time 10-12 2.5 x 10-4 cm-3 3.4 x 104 km 44 days 10-15 25 cm-3 3.4 x 107 km 120 years 10-19 Interstellar space 0.25m-3 2.3 x 103 AU 1.2 x 106 years Redhead, P. A. Review Literature And Arts Of The Americas 213–226 (1958). Three main types of pumps Positive displacement pumps use a mechanism to repeatedly expand a cavity, allow gases to flow in from the chamber, seal off the cavity, and exhaust it to the atmosphere. Momentum transfer pumps, also called molecular pumps, use high speed jets of dense fluid or high speed rotating blades to knock gas molecules out of the chamber. Entrapment pumps capture gases in a solid or adsorbed state. This includes cryopumps, getters, and ion pumps. Pumping Ranges people.rit.edu/vwlsps/LabTech/Pumps.pdf Pumping Ranges people.rit.edu/vwlsps/LabTech/Pumps.pdf Viscous vs. Molecular Flow Regimes The gas in a vacuum system can be in a viscous state, in a molecular state or in a state which is intermediate between these two. When a system is brought from the atmospheric pressure to "high vacuum", the gas in the system goes through all these states. The mean free path of the gas molecules is very small at atmospheric pressure so that the flow of the gas is limited by its viscosity. At low pressures where the mean free path of the molecules is similar to the dimensions of the vacuum enclosure, the flow of the gas is governed by viscosity as well as by molecular phenomena; this is the intermediate flow. At very low pressures where the mean free path is much larger than the dimensions of the vacuum enclosure, the flow is molecular. Viscous > 10-4 Molecular < 10-6 Rotary Pump http://www.quorumtech.com/Products/RV5PUMP.jpg Rotary Pump Check oil level once a month Ensure the pump is able to cool Mechanical pumps can introduce noise in STM Turbomolecular Pump http://www.varianinc.com http://www.pfeiffer-vacuum.com Turbomolecular Pump Turbo pumps utilize a stack of turbine blades which rotate at very high speed (of order 50,000 rpm) to move gas from the inlet port to the exhaust port. Turbo pumps are very effective at low pressures (<10 torr), essentially in the molecular flow regime in which gas densities are so low that the molecules collide with chamber walls far more often than with each other. As a result, turbo pumps can achieve chamber base pressures of 10-9 torr or below. However, the high packing of fan blades and the high rotation speed of the turbo pump make it ineffective at higher pressures, where fluid (viscous) flow dominates. Powering a turbo pump alone at atmospheric pressure will barely cause the blades to rotate. THEREFORE TURBOS ARE BACKED BY ROTARY PUMPS Stalling of turbo pump Ion Pumps 1 Permanent magnets 2 Pump envelope 3 Titanium cathodes 4 Anode cell array 5 Positive high voltage lead http://www.thermionics.com/ip_too.htm Ion Pumps Sputter ion pumps operate by ionizing gas within a magnetically confined cold cathode discharge. The events that combine to enable pumping of gases under vacuum are: Entrapment of electrons in orbit by a magnetic field. Ionization of gas by collision with electrons. Sputtering of titanium by ion bombardment. Titanium gettering of active gases. Pumping of heavy noble gases by ion burial. Diffusion of hydrogen and helium into titanium. Dissociation of complex molecules into simple ones for pumping ease, e.g., CH4 breaks down into C and 2H2. Hydrogen is pumped separately. Carbon is no longer part of the residual gas and resides in solid form. 3 -7 kV range. 5 kV No moving parts or oil: no maintenance or vibration http://www.thermionics.com/ip_too.htm Titanium Sublimation Pumps (TSPs) Resistively heat Ti metal Thin layer of Ti on chamber walls This reacts with O2 etc and pumps chamber N.B. Sample areas must be shielded! Titanium Sublimation Pumps (TSPs) Can’t pump un-reactive gases: Noble gases, Argon TSP are not flashed while at cryogenic temperatures. This leads to: cryogen boil off due to high temperature outgassing from the TSP. This will then adhere to any cold surface, including the sample. http://www.aiv.it/?q=content/gettering-and-ion-pumping Cyropumping N2(L) He(L) Overtime the surface area becomes saturated and pumping speed drops to zero Systems needs to be recharged by warming and pumping outgas by other means During He(L) fills H2 and CO are outgassed Dawson, J. & Haygood, J. Cryopumping. Cryogenics (1965) Useful Websites Folder in the general user folder on the N drive http://www.pfeiffer-vacuum.com/know-how http://www.aip.org/avsguide/refguide/workingpress.html http://www.duniway.com/images/pdf/pg/ion-pump-op-troubleshoot.pdf Ion pump working http://www.gammavacuum.com/operation.asp Bake out and outgassing Even once all this is in place, you still cannot achieve UHV. The problem is that a lot of gas molecules are adsorbed to the walls of your vacuum chamber. Once you lower the pressure, these molecules are sometimes desorbed and pumped away but this is a very slow process. The way to accelerate this (exponentially!) is to increase the temperature. Therefore, one has to perform a so-called bakeout of the system in which the entire UHV system is heated to a high temperature, typically higher than 100 degrees Celsius. Heating the system up to this temperature can take a long time, especially if you have thermally well-insulated parts inside which are only heated by radiation. The same is true for cooling it down again, so the whole procedure can take a more than a day. When cooling down, it is a good idea to operate any filaments (and devices containing filaments, such as ion gauges, sputter guns, titanium sublimation pumps, residual gas analyzers and so on) at a higher temperature than you usually use under normal operation, in order to avoid massive desorption of the adsorbed rest gas later. This procedure is called outgassing. Useful Websites http://www.aip.org/avsguide/refguide/workingpress.html