Lab - Testing Properties of Substances
advertisement

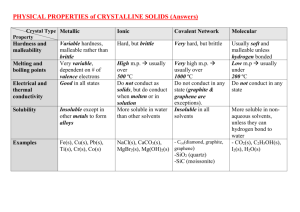
Grade 10 Science Inquiry: ____/ 20 Names: ____________________________ Communication: ____/10 Testing Properties of Substances Purpose: to test the physical properties (solubility and electric conductivity) and the chemical properties (reaction with an acid) of several substances. Materials: safety glasses, scoopulas, 100 mL beaker, tap water, stirring rod, conductivity apparatus, magnesium, hydrochloric acid solution, medium test tubes, wooden splint, limewater solution -sodium chloride (salt) -naphthalene (mothballs) -calcium carbonate (chalk) -copper (II) sulfate -potassium bromide -glucose -sucrose (table sugar) Method: Part A – Solubility 1) Obtain a 100 mL beaker, a stirring rod, and a scoopula full of one of the substances from the list above. Describe the solid substance in the observation table. 2) Fill the beaker one-third full with tap water, then add the substance to the water, and stir. Record the solubility of the substances in the observation table as “soluble” or “insoluble”. 3) If the substance is soluble in water, then go on to Part B and test its conductivity in solution. If the substance is insoluble in water, then go on to part 4). 4) Rinse the beaker and repeat Steps 1-3 for all the substances on the observation table. Part B – Conductivity 1) Take the solution to a conductivity apparatus and test whether or not the solution conducts electricity (the light will come on if the solution completes the circuit). 2) Record the ability of the substance to conduct electricity when dissolved in water as “electrolyte” or “nonelectrolyte”. Part C – Reaction with Acid 1) Fill a medium test tube one quarter full with hydrochloric acid. Obtain a small piece of magnesium. Describe the substances in the observation table. Light the Bunsen burner, and prepare a flaming splint. Turn off your Bunsen burner. 2) In the meantime, add the magnesium to the test tube of hydrochloric acid. Record your observations. Wait about 30 seconds, then insert the flaming splint into the test tube with arms outstretched in front of you. Record your observations. 3) Fill a medium test tube one quarter full with hydrochloric acid. Fill a second test tube one quarter full with lime water. Obtain a scoopula full of calcium carbonate. Describe the substances in the observation table. 4) Add the calcium carbonate to the test tube containing hydrochloric acid. Immediately, pour the gas formed by the reaction into the limewater solution as your teacher has demonstrated (the gas is invisible, so you have to have faith that it is “pouring” into the limewater). Record your observations. Group Names: ___________________________________________ Observations: These will be handed in on the same day as the lab, and the mark will be shared. Part A – Solubility (2 marks) Substance sodium chloride Physical Properties of Solid Solubility in H2O calcium carbonate potassium bromide naphthalene copper (II) sulfate glucose sucrose Part B – Conductivity When Dissolved in Water (1 mark) Substance Conductivity Part C – Reaction with Acid (5 marks) Substance Physical properties magnesium flaming splint: hydrochloric acid Substance calcium carbonate Observations upon mixing reaction: Physical properties Observations upon mixing reaction: limewater: hydrochloric acid Name: _______________________________ Analysis: These questions will be answered in class the day after the lab, in a “test-like” format. The correct observations will be projected on the screen for reference. Answer the questions in the space provided using full sentences. 1) Explain why solubility and electrical conductivity are physical properties, while reaction with acid is a chemical property. (2) Part A: Solubility 2) Were there any patterns associated with whether a compound dissolves in water? (1) Part B: Conductivity in Water 3a) The compounds tested in Part B that were electrolytes contained sodium, potassium or copper. What kinds of elements are these (metals or non-metals). (1) b) The compounds tested in Part B that were nonelectrolytes were made up entirely of the elements carbon, oxygen and hydrogen. What kinds of elements are these (metals or non-metals)? (1) Part C: Reaction with Acid 4) What gases were tested for in part C of this experiment? What does the formation of a gas indicate about the two experiments in Part C? (3) Conclusion: Use the answers to the above questions to write a short conclusion to this lab. It should be four sentences long (one sentence to summarize each question from the analysis). (4)