Reactions of hydrochloric acid
advertisement

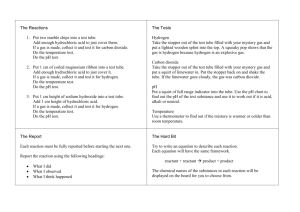
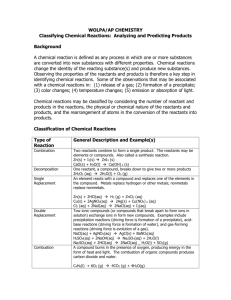
Reactions of hydrochloric acid Aim: to investigate some common reactions of hydrochloric acid Materials: Collect the following for your group…. 0.1 M hydrochloric acid (HCl) 1 M hydrochloric acid (HCl) 0.1 M sodium hydroxide (NaOH) 1 cm magnesium ribbon (Mg) Solid calcium carbonate (CaCO3) Solid copper oxide (CuO) Stopper and delivery tube Safety glasses limewater methyl violet indicator universal indicator colour chart spatula 4 test tubes Test tube rack lab coat Procedure: 1. a) Add one drop of universal indicator to a test tube containing 20 drops of 0.1 M hydrochloric acid b) Add 0.1 M sodium hydroxide drop wise and record any colour changes. c) Explain your observations and write an equation for the reaction that has occurred. Observation: ____________________________________________ _____________________________________________ Explanation: _____________________________________________ _____________________________________________ Reaction: _____________________________________________ 2. pH: a) Add one drop of methyl violet indicator to a test tube containing 20 drops of 0.1 M HCl. Record the pH of the solution. What does this tell you about the strength of hydrochloric acid? ____________ Strength of acid _________________________________________ b) Add 10 ml of water to the test tube and then test the pH again. What do you notice? ___________________________________ 3. Add about 1 ml of 1M hydrochloric acid to a test tube containing a spatula full of solid calcium carbonate. Test the gas evolved with limewater. Observation: ____________________________________________ _____________________________________________ Reaction: 4. _____________________________________________ Add about 5 ml of 1M hydrochloric acid to a spatula full of solid copper oxide in a test tube. Shake the test tube and allow to stand for 5 minutes. Observation: ____________________________________________ _____________________________________________ Reaction: 5. _____________________________________________ Add about 10 ml of 1M hydrochloric acid to a test-tube containing a 1 cm strip of magnesium ribbon. Use an inverted test tube to collect the gas evolved and test it with a flame. Observation: ____________________________________________ _____________________________________________ Reaction: _____________________________________________ Conclusion: _______________________________________________________ _______________________________________________________ _______________________________________________________ _______________________________________________________ _______________________________________________________