SCH4U1 - Chatt
advertisement

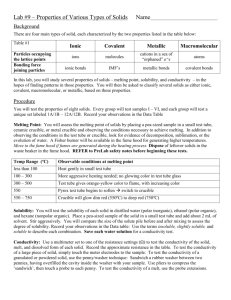
SCH4U1 UNIT 2 PROPERTIES OF SOLIDS Purpose: 1) To study the physical properties of a variety of solids. 2) To identify unknown solids by their physical properties. Materials: watch glasses scoopula / scalpel wire mat ring clamp bunsen burner flint lighter silicon, granite, aluminum, sodium electrical conductivity apparatus retort stand test tube stopper test tube rack fume hood unknown substances A - C ohmmeter Part 1: Method: Carry out the following steps for the three unknown substances only. 1. Using the hand waving method note the odour of each unknown solid substance. Record findings as either: strong, mild or no odour. 2. Obtain a very small sample of each substance on a watch glass. 3. Place a few crystals of each of the three solids on a ceramic fibre wire mat (be sure to keep track of the position of each substance) . Place the wire mat on a ring clamp attached to a retort stand in the fume hood. Heat the substances gently. Record the relative melting points of the three substances (low, medium, high, does not melt) 4. Add distilled water to a clean dry test tube to a height of about 3 cm. Then add a few crystals of one of the solids to the test tube. Stopper the test tube and shake. Note the relative solubility of the substances. Rinse out the test tube and repeat step 4 for the other two solids. 5. Place 10 - 20 mL of water in a 50 mL beaker and add a scoop of the unknown substance. Stir with a glass rod to dissolve and test the electrical conductivity of the resulting solution . Note the relative conductivity and repeat for the other 2 solids. Observations: Create a table with the following headings in your lab book to record your data. Sample Odour Melting Point Solubility Conductivity in Solution Analysis: 1. Complete a chart with the following columns: a) Unknown: A – Z b) Identity : Determine the identity of each unknown sample by comparing the chemical formulae and the observed physical properties. c) Type of Substance: For each of these compounds, state whether it is an atomic, molecular, network, metallic or ionic solid. d) Force(s) of Attraction: State the types of attraction present in each of these compounds which explain the observed characteristics. 2. a) Using a diagram, explain how one of these three unknown substances can conduct electricity in an aqueous solution. b) Would you expect this substance to conduct as a solid? Explain why or why not. Part 2: Observations: Create a table like with the following headings: Substance Hardness Resistance (ohms) Method: Carry out these steps for silicon (Si), aluminum (Al), quartz (SiO2) and sodium (Na). 1. Try to scratch the surface of each solid with a scalpel. Note the relative hardness of the solids. 2. Test the electrical conductivity of the solids using an ohmmeter. Note: Resistance (in ohms) is inversely proportional to conductivity). Analysis: 1. For each of the substances used in Part 2, state whether it is an atomic, molecular, network, metallic or ionic solid. 2. a) Explain the difference in conductivity between aluminum and quartz (i.e. explain why each substance conducts or does not conduct).. b) Explain the difference in conductivity between sodium and silicon. 3. a) Explain the difference in hardness between sodium and aluminum. b) Explain the difference in hardness between silicon and aluminum.