Protocol Summary
advertisement

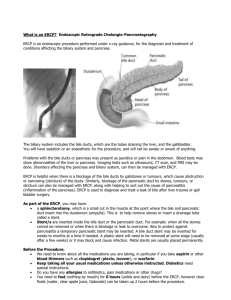
MRCP in the Assessment of Gallstone Disease PROTOCOL VERSION 1.4 LAST UPDATED 13 JULY 2014 Contents Key Contacts ..................................................................................................................................... 3 Protocol Summary............................................................................................................................ 4 Abstract .................................................................................................................................................. 5 Introduction............................................................................................................................................ 7 STUDY HYPOTHESIS................................................................................................................................. 9 OBJECTIVES ........................................................................................................................................... 10 DESIGN AND METHODS ........................................................................................................................ 11 Eligible Patients ................................................................................................................................. 11 Patient Identification ........................................................................................................................ 11 Study Period ...................................................................................................................................... 12 Eligible Centres.................................................................................................................................. 12 Data Extraction ................................................................................................................................. 12 Trainees’ Roles .................................................................................................................................. 13 OUTCOME MEASURES AND STANDARDS ................................................ Error! Bookmark not defined. DATA COLLECTION ................................................................................................................................ 14 DEFINITIONS.......................................................................................................................................... 15 STATISTICAL ANALYSIS ............................................................................. Error! Bookmark not defined. REFERENCES .......................................................................................................................................... 16 APPENDIX ................................................................................................. Error! Bookmark not defined. MAGD STEERING TEAM List of Key Contacts Miss Judith Ritchie CRUK Clinical Research Fellow, Faculty of Medicine, University of Leeds, UK jeritchie@doctors.org.uk Dr Emma Nofal Core Surgical Trainee, Yorkshire and the Humber Deanery, Sheffield, UK emn500@gmail.com Jonathan R L Wild Higher Surgical Trainee, Yorkshire and the Humber Deanery, Sheffield, UK jonathanrlwild@gmail.com Raj Prasad Consultant General Hepatobiliary and Transplant Surgeon Leeds Teaching Hospitals NHS Trust This protocol was developed on behalf of the committee members of the South Yorkshire Surgical Research Collaborative: Judith Ritchie, Emma Nofal, Matthew Lee, Jonathan Wild, Roxanna Zakeri, Victoria Proctor, Matthew Rigby, Thomas Drake, Tim O’ Connor, Charlotte Gunner, Sarah Daniels. Protocol Summary GENERAL INFORMATION Short title Full title Sponsor Chief Investigators Website Email Co-ordinating Centre STUDY INFORMATION Aim of the study Study Design Primary Outcomes Secondary Outcomes STUDY TIMELINES Pilot date Main study period End of Study Definition Data submission (live process) Data analysis Results available Paper submission MGD Study The Role of MRCP in Gallstone Disease South Yorkshire Surgical Research Collaborative Judith Ritchie, Emma Nofal, Jonathan Wild To look at current investigative practice for suspected choledocholithiasis in order to investigate the role of MRCP in the assessment of patients presenting with suspected ductal stone disease and its impact on surgical management Retrospective Observational Service Evaluation Determining variation in investigative practice in patients with suspected ductal stones Detection and miss rate of common bile duct stones for different practices (MRCP, USS, CT, ERCP, EUS, OTC) All-cause readmission rates Post-operative (complications from retained common bile duct stones (pancreatitis, obstructive jaundice, pain, other complications)) within 6 months postoperation Time from presentation to management (ERCP, LC ±OTC) Time from presentation to investigation (USS; MRCP, EUS, OTC±ECBD, PTC) Length of hospital stay, duration of surgery Pre-operative patient factors (demographics, admission type, level of health/ASA grade and fitness for surgery, contraindications to MRCP) Cost analysis of use of services (blood tests, USS, CT, EUS, MRCP, OTC) May 2014 SYSuRG September 2013 November 2014 November 2014 December 2014 January 2015 March 2015 Abstract BACKGROUND The benefit of MRCP in identifying ductal stones has been widely reported in case series of patients with gallstone disease. Clinical assessment of patients with gallstones varies widely across centres for a number of reasons.. This study aims to look at use of MRCP in centres across the UK, to determine current practices and their variations, and how these affect clinical outcome. METHODS The study consists of 2 parts: a retrospective multi-centric service evaluation and a consultant surgeon questionnaire. The retrospective study will capture data over a 4 week period across teaching and district general hospitals that have an adult acute surgical take, an ERCP facility and resident consultant surgeons that routinely do cholecystectomies. A cohort of patients presenting with gallstone disease with suspected ductal stones will be identified from operative and ERCP lists. Clinical information will be collected including past medical history and ASA grade, presence of sepsis, blood work, imaging, previous biliary history and previous interventions. Outcome data will include length of acute admission stay, timescale and details of investigations, operative details (cholangiogram, CBD exploration, post-operative ERCP) and postoperative complications (readmission, identification of retained stones). A rudimentary cost analysis shall be performed. This study will also include a questionnaire sent to UK surgeons performing cholecystectomies regarding their management of these patients and the use of MRCP in their practice. OUTPUT This study shall generate a large and informative dataset regarding current clinical practice for suspected choledocholithiasis. The results of this study will be presented at ASGBI, AUGIS and BSG conference and will be prepared for publication. Introduction Gallstone related disease is a significant cause of morbidity with a major impact on modern day healthcare. The prevalence of common bile duct stones in symptomatic gallstone disease has been estimated to be between 10-20% of cases(Tazuma 2006). Ductal stones can present with pain and deranged liver function tests, or occasionally may be an incidental finding in asymptomatic patients. Detection and removal of choledocholithiasis is important as they can result in stone-related complications such as obstructive jaundice, ascending cholangitis, liver abscess and gallstone pancreatitis. Therefore, clinical judgement is paramount, and it should guide appropriate use of diagnostic modalities as much as management. Diagnostic imaging of the biliary tree can technically be achieved using both invasive and non-invasive measures. Non-invasive imaging includes ultrasound, CT and MRI. More invasive measures include cholangiogram of the biliary system, which can be performed endoscopically through cannulation of the ampulla at endoscopy (ERCP) and percutaneously through cannulation of the intrahepatic biliary system (PTC), or intraoperatively at time of cholecystectomy. Ultrasound imaging is the first line recommended imaging technique in clinically suspected gallstone disease and also for suspected choledocholithiasis (Williams 2008), and can reliably detect stones within the gallbladder. However, ultrasound is not a reliable diagnostic test to justify the use of more invasive measures such as common bile duct exploration. It has a high sensitivity and specificity for detecting intrahepatic duct dilatation (Stott, Farrands et al. 1991), but a poor sensitivity but a high specificity for detecting ductal stones (Stott, Farrands et al. 1991; Varghese, Liddell et al. 1999; Varghese, Liddell et al. 2000). Furthermore, the presence of intrahepatic duct dilatation on ultrasound does not correlate reliably with the presence of ductal stones at intraoperative cholangiogram (Stott, Farrands et al. 1991). Magnetic cholangiopancreatography is a non-invasive imaging technique that gives three dimensional images of diagnostic clarity of the biliary system similar to those achieved by endoscopic cholangiogram. MRCP has been shown to give comparable diagnostic results to ERCP (Adamek, Albert et al. 1998; Shanmugam, Beattie et al. 2005) with a high sensitivity and specificity for choledocholithiasis (Taylor, Little et al. 2002; Topal, Van de Moortel et al. 2003; Shanmugam, Beattie et al. 2005) without the associated morbidity. The ability to detect choledocholithiasis by CT depends upon the constitution of the gallstones. Calcified stones will be readily visible whereas pure cholesterol stones hypoattenuate against bile (Brakel 1990)which can make them impossible to detect, particularly by non-contrast CT. CT imaging has a low sensitivity relative to MRCP and cholangiography, but this is significantly improved with contrast enhancement versus non/contrast enhancement (Soto, Alvarez et al. 2000). MRCP has a similar diagnostic capacity as cholangiography for choledocholithiasis (Varghese, Liddell et al. 2000), biliary structures and malignant pancreatic pathology (Rösch, Meining et al. ; Hekimoglu, Ustundag et al. 2008) and has become the preferred non-invasive test for detecting ductal stones (Paul 2006; Williams 2008). However there are technical contraindications to MRI, in particular indwelling metallic implants such as pacemakers, aneurysm coils and nerve stimulators, and it may not be tolerated by patients with claustrophobia(Kaltenthaler E 2004). ERCP has been shown to have a high sensitivity and specificity for detecting ductal stones (Varghese, Liddell et al. 1999). Its associated rates of morbidity and mortality (Andriulli 2007; Williams, Taylor et al. 2007) such as perforation and post-ERCP pancreatitis do not make this an acceptable diagnostic test, and ERCP should be used solely for therapeutic purposes (Williams 2008; Royal College of Surgeons of England 2013). EUS generates good images of the biliary system, on which stones are visualised as hyperechoic foci. It has been demonstrated to be as sensitive and specific for choledocholithiasis to MRCP (Verma, Kapadia et al. 2006). Management options for choledocholithiasis include endoscopic via therapeutic ERCP to remove stones plus/minus sphincterotomy where there is a high likelihood of recurrent stones or biliary stenting to bypass impacted stones and restore biliary flow. Surgical management includes exploration of the common bile duct and stone extraction at time of cholecystectomy, with pre- or postoperative ERCP where stones are suspected. Service provision for ERCP requires dedicated endoscopy services and experienced endoscopists trained in ERCP which can make ready accessibility to its service difficult. Similarly, common bile duct exploration requires a considerable level of expertise that will not be available across the cohort of surgeons performing cholecystectomies. There is very little robust evidence regarding the efficacy of endoscopic versus surgical common bile duct exploration. Existing evidence suggests there is less chance of missed stones following open CBD exploration but no difference between laparoscopic CBD exploration and ERCP (Dasari, Tan et al. 2013), with comparable morbidity and mortality. Current guidelines on management of common bile duct stones recommend abdominal ultrasound as a first line investigation along with blood tests including liver function (Williams 2008). EUS or MRCP is recommended for further investigation where choledocholithiasis is suspected, depending on patient suitability, accessibility to resources and expertise (Williams 2008). Clinical practice in investigating and managing suspected common bile duct stones varies between centres, influenced by surgeons' practice and local expertise (Poulose, Phillips et al. 2011), access and availability of resources (Williams, Taylor et al. 2007) and individual patient factors. This study aims to explore this variation in clinical practice, and more specifically determine whether it has any impact on patient outcomes. STUDY HYPOTHESES MRCP has made a significant impact on the detection of ductal stones or other ductal pathology in the surgical patient cohort. Use of non-invasive diagnostic modalities is associated with less morbidity. MRCP is associated with lower morbidity, a lower incidence of missed CBD stones and better patient outcomes. Variations in clinical practice may affect influence patient outcomes. Investigation and treatment of ductal stones in clinical practice is affected by local access and availability of resources. OBJECTIVES The aim is to gain insight into the practices used in the surgical assessment of the biliary system in general surgery units across the country and whether this has any impact on surgical outcomes. In particular, this study wants to determine the impact that the non-invasive imaging modality MRCP has had on patient care. The primary objective is to determine the detection rate and miss rate of ductal stones according to the approach taken to investigation and management. The secondary objectives are: To determine time from presentation to each imaging modality (USS, CT, EUS, MRCP) and the time from presentation to definitive treatment (ERCP, surgery and OTC± CBD exploration), or non-surgical interventions in those unfit for surgery e.g. ERCP and stent, PTC and stent) To determine the effect of other surgical outcomes including length of operation, length of hospital stay (for surgery taking place during the presentation episode) To determine the all-cause 30 day complication and readmission rate following surgery and determine how these rates correlate to preoperative assessment. To determine the cost of investigations. DESIGN AND METHODS This study is comprised of two parts. The first part is a retrospective multi-centric service evaluation. The second part is a postal questionnaire for UK consultant surgeons who performed the cholecystectomies included in the study period. The questionnaire aims to explore each consultant’s practice in assessment and management of these patients and the reasons behind this. Eligible Patients Adult patients presenting to general surgical units across the country with a diagnosis of gallstone disease with suspected ductal stones who have undergone cholecystectomy or ERCP during the study period (4 week period in FebruaryMarch 2013), namely: Symptomatic gallstones with deranged liver function tests Obstructive jaundice with low suspicion of cancer (painful/painless) Gallstone pancreatitis Patient Identification Eligible Patients who have undergone cholecystectomy or ERCP during the study period will be included. These patients will be identified by scrutiny of the operative management systems (cholecystectomy codes) and endoscopy data management systems that are in use at each participating hospital Not Eligible Patients under the age of 18 Data extraction forms comprise an eligibility form which would determine whether further data extraction and follow-up is warranted. Study Period Pilot The study shall be piloted within Sheffield over a one week period in July 2014. This pilot period will allow the feasibility and applicability of the study protocol and data extraction. The study shall be registered with the clinical effectiveness and audit units in each centre prior to carrying out the pilot. Any alterations to the study protocol or data extraction process will be made following evaluation of this pilot period. Main Study The main study will recruit over 4 weeks. The study is projected to start from September 2014. Eligible Centres Surgical centres across the country will be invited to take part. Eligible centres include: Centres with an acute surgical admissions unit. Centres with consultant surgeons routinely performing elective and emergency cholecystectomies. Centres with endoscopy units that run regular ERCP lists. Data Extraction The data extraction form will capture important demographic and clinical details from these patients including: Nature of presentation (elective, emergency) General health with ASA grade. Drug history. Previous biliary history, investigation and intervention Contraindications to imaging (pacemaker/metalwork, claustrophobia etc). The study will also capture information regarding the surgical workup Clinical parameters (signs of biliary sepsis) Blood work (deranged LFTs (new/not new/no previous to compare) Imaging (USS, CT, MRCP, EUS, other) Preoperative interventions (ERCP, PTC) Details regarding the surgical outcome shall be sought: Cholecystectomy (index, scheduled/hot list, elective, delayed) On table cholangiogram ± bile duct exploration Grade of surgeon Consultant name Length of surgery Definitive treatment if no surgery (and reason) Finally details regarding the post-operative outcomes shall be gathered, namely: Perioperative complications Postoperative complications during surgical episode Length of acute admission stay Length of surgical stay Readmission with post-operative complications following discharge from surgical episode Further investigations and interventions Trainees’ Roles Interested trainees at each centre should nominate one trainee as the lead investigator, and identify an appropriate consultant surgeon at their unit who is happy to be the consultant lead for their centre. The lead investigator must take responsibility for registering the study through their hospital’s clinical audit and effectiveness unit according to their local policy. Proof of consultant lead and registration with the audit office will need to be provided to SYSuRG before the study begins. Ongoing support will be available from SYSuRG for trainees taking part in the study. DATA COLLECTION Data should be collated for each centre electronically. Leeds University is hosting an online encrypted secure database. An account shall be made for a contact at each participating centre into which to input data. All data should be recorded according to the policy of their hospital’s clinical audit unit policy. Hospitals should fill out the relevant Data Protection forms that are available from Information Governance. All data should be anonymised at point of entry into electronic database. No patient identifiable information should be entered into the database. Each centre is asked to therefore generate a study number for each eligible patient. SYSURG MAGD team can help, advise or answer any questions regarding any aspect of the above on request. AUTHORSHIP All those coordinating and contributing in data collection will be listed as authors in any publications arising from this work, after the relevant members of the project steering committee. Therefore all those actively contributing to the project would be listed in Pubmed as an author to the paper in a Pubmed linked journal. DEFINITIONS MRCP: Magnetic resonance cholangiopancreaticogram ERCP: Endoscopic retrograde cholangiopancreaticogram EUS: Endoscopic ultrasound Deranged liver function tests: Bilirubin above Alkaline phosphatase above GGT above ALT above REFERENCES Adamek, H. E., J. Albert, et al. (1998). "A prospective evaluation of magnetic resonance cholangiopancreatography in patients with suspected bile duct obstruction." Gut 43(5): 680-683. Andriulli, A., Loperfido, S, Napolitano, G, Niro, G, Valvano, MR, Spirito, F, Pilotto, A, Forlano, R (2007). "Incidence Rates of Post-ERCP Complications: A Systematic Survey of Prospective Studies." Am J Gastroenterology 102(8): 1781-1788. Brakel, K., Lameris, JS, Nijs, HGT, Terpstra, OT, Steen, G, Blijenberg, BC (1990). "Predicting Gallstone Composition with CT: In Vivo and In Vitro Analysis." Radiology 174: 337-341. Dasari, B. V., C. J. Tan, et al. (2013). "Surgical versus endoscopic treatment of bile duct stones." Cochrane Database Syst Rev 12: CD003327. Hekimoglu, K., Y. Ustundag, et al. (2008). "MRCP vs ERCP in the evaluation of biliary pathologies: Review of current literature." Journal of Digestive Diseases 9(3): 162-169. Kaltenthaler E, B. V. Y., Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al. (2004). "A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography." Health Technol Assess 8(10). Paul, A., Millat, B, Holthausen, U, Sauerland, S, Neugebauer, EAM, Verthou, JC, Brambs, H-J, Dominguez-Munoz, JE, Goh, P, Hammerstrom, LE, Lezoche, E, Perissat, J, Rossi, P, Rithlin, MA, Russell, RCG, Spinelli, R, Tekant, Y (2006). EAES Guidelines for Endoscopic Surgery. Twelve Years Evidence Based Surgery in Europe. E. Neugebauer, Sauerland, S, Fingerhut, A, Millat, B, Buess, G. Germany, Springer. Poulose, B. K., S. Phillips, et al. (2011). "Choledocholithiasis Management in Rural America: Health Disparity or Health Opportunity?1." The Journal of surgical research 170(2): 214-219. Rösch, T., A. Meining, et al. "A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures." Gastrointestinal Endoscopy 55(7): 870-876. Royal College of Surgeons of England, A. (2013). "Commissioning Guide: Gallstone Disease." 1-13. Shanmugam, V., G. C. Beattie, et al. (2005). "Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging?" The British Journal of Radiology 78(934): 888-893. Soto, J. A., O. Alvarez, et al. (2000). "Diagnosing Bile Duct Stones." American Journal of Roentgenology 175(4): 1127-1134. Stott, M. A., P. A. Farrands, et al. (1991). "Ultrasound of the common bile duct in patients undergoing cholecystectomy." Journal of Clinical Ultrasound 19(2): 73-76. Taylor, A. C. F., A. F. Little, et al. (2002). "Prospective assessment of magnetic resonance cholangiopancreatography for noninvasive imaging of the biliary tree." Gastrointestinal Endoscopy 55(1): 17-22. Tazuma, S. (2006). "Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic)." Best Pract Res Clin Gastroenterol 20(6): 1075-1083. Topal, B., M. Van de Moortel, et al. (2003). "The value of magnetic resonance cholangiopancreatography in predicting common bile duct stones in patients with gallstone disease." British Journal of Surgery 90(1): 42-47. Varghese, J. C., R. P. Liddell, et al. (2000). "Diagnostic Accuracy of Magnetic Resonance Cholangiopancreatography and Ultrasound Compared with Direct Cholangiography in the Detection of Choledocholithiasis." Clin Radiol 55(1): 25-35. Varghese, J. C., R. P. Liddell, et al. (1999). "The diagnostic accuracy of magnetic resonance cholangiopancreatography and ultrasound compared with direct cholangiography in the detection of choledocholithiasis." Clin Radiol 54(9): 604-614. Verma, D., A. Kapadia, et al. (2006). "EUS vs MRCP for detection of choledocholithiasis." Gastrointestinal Endoscopy 64(2): 248-254. Williams, E., Green, J, Beckingham, I, Parks, R, Martin, D, Lombard, M (2008). "Guidelines on the management of common bile duct stones (CBDS)." Gut 57: 1004-1021. Williams, E. J., S. Taylor, et al. (2007). "Are we meeting the standards set for endoscopy? Results of a large-scale prospective survey of endoscopic retrograde cholangio-pancreatograph practice." Gut 56(6): 821-829.