REACTION RATES LAB - misskaleyhanson
advertisement

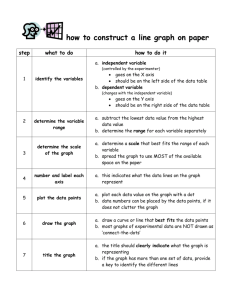
Name: _______________________ REACTION RATES LAB Go to my website http://misskaleyhanson.wikispaces.com Click on Science 9 Unit 2 Reaction Rates Lab You will be investigating how TEMPERATURE, PARTICLE SIZE, and CONCENTRATION affect rates of reaction. At the top of the page there are three tabs for each of the above. Do each of the tabs. First click on the TEMPERATURE tab. Then, to access the interactive, click on the graphic on the page to launch it. Use the interactive by following the instructions below and answer the questions for each part. TEMPERATURE You will investigate how changes in temperature affect the reaction rate of an antacid tablet dissolved in water. Problem: How does temperature affect the rate of reaction? Make a HYPOTHESIS for the problem: Follow the Procedure below: 1. Read the safety poster. 2. Click on the equipment cupboard. 3. Select the equipment that you will use for this experiment (take out the beaker, hot plate, thermometer, antacid, timer.) ***note the reset button in the right hand corner is used to return settings back to normal ***** 4. Place the ice in the beaker of water and record the temperature in the data table below. 5. Place the antacid tablet in the beaker, start the timer by clicking on it. Stop the timer once the antacid is dissolved. Record how long this took in your data table. 6. Reset the lab equipment and set the hot plate to 1. Drop in the antacid and record the time it takes to dissolve in the data table. 7. Repeat this process for hot plate settings of 2, 4, 6, 8, and 10. Name: _______________________ Observations Data Table x axis y axis Temp Time (s) (°C) Conclusion 1. Which factor is the independent variable (what you change)? 2. Which factor is the dependent variable (what changes as a result of changing the manipulated variable)? 3. What variables are the control variables (do not change)? 4. Initially, what happened to the time it took the antacid tablet to dissolve as you increased the temperature? 5. Between which two temperatures was there the fastest reaction rate? 6. Write a general statement that would answer the question stated in the problem. 7. At what temperature did the time it takes for the antacid to dissolve begin to increase? Analysis and Extension 1. Make a graph of your results. Give the graph a title and label both axes. Name: _______________________ 2. Why do you think your graph is not linear (a straight line)? In other words, why does the time it takes the antacid to dissolve not continue to decrease as the temperature increases. PARTICLE SIZE You will investigate the change in reaction rate when varying sizes of zinc particles are reacted with sulfuric acid. Problem: How does particle size affect the rate of reaction? Write you HYPOTHESIS for the Problem above: Follow the Procedure below: 1. 2. 3. 4. 5. Read the safety poster describing the safe handling of acids. Place the 8.0 mm Zn sample in the beaker of H2S04 acid. Record the time it takes for the sample to dissolve, in the data table. Click the Reset button and choose Reset Lab Equipment. Repeat these steps for each successively smaller sample, recording the dissolving time for each in the data table. 6. Return your equipment to the cupboard when you are finished Observations: Data Table x axis y axis Avg. size of particles of Zn (mm) Time (s) 8.0 4.0 2.5 1.5 0.5 Name: _______________________ Conclusion: 1. What evidence is there that a chemical reaction is taking place? 2. Which variable is the independent variable? 3. Which variables are control variables? 4. How does the size of the particle affect the rate of reaction? Extension: 1. Create a graph of your results. Give the graph a title and label both axes. CONCENTRATION You will investigate the reaction rates of varying concentrations of sulfuric acid combined with magnesium metal. Problem: How does concentration affect the rate of reaction? Write a HYPOTHESIS for the Problem above: Procedure 1. Read the safety poster describing the safe handling of acids. 2. Open the lab supply cupboard and place your materials on the lab bench. 3. Set your H2SO4 Concentration level to 5%. 4. Use the forceps to place the magnesium ribbon in the beaker. 5. Use the timer to determine the time it takes for the magnesium ribbon to completely dissolve. 6. Record the concentration and time in the data table. Name: _______________________ 7. Use the Reset button to reset the lab equipment. 8. Repeat these procedures for concentrations of 10%, 20%, 30%, 40%, and 50%. Record the information in the data table. Observations: x axis CONC H2SO4 % 5 10 20 30 40 50 y axis Time (s) Conclusion: 1. What evidence is there that a chemical reaction is taking place? 2. Which variable is the independent variable? 3. Which variables are control variables? 4. How does the concentration of the acid affect the rate of reaction? Extension: 1. Suggest a reason why the time it takes for the magnesium ribbon to dissolve does not continue to decrease. 2. Create a graph of your results. Label the axis and give it a title.