Degrees of Separation
advertisement

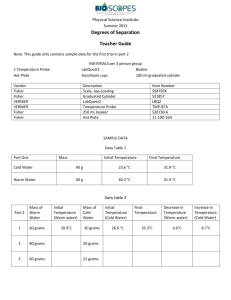
Physical Science Institute Summer 2013 Degrees Of Separation To this point, our investigation of mechanical energy has been limited to potential and kinetic energy. The Law of Conservation of Energy states that energy can transform from one form to another. But this model is not limited to potential and kinetic energy. This activity will allow us to observe another form of energy know as thermal energy. MATERIALS 1 Temperature Probe Hot Plate LabQuest2 Styrofoam cups Beaker 100 ml graduated cylinder PROCEDURE PART 1: Mixing Warm and Cool Water 1. Measure out 40 grams of water at room temperature. Use the fact that water has a density of 1g/cm 3. This allows you to measure the mass using a graduated cylinder. Record this temperature in data table 1. 2. Heat some water on the hot plate in the beaker. 3. Measure an equal mass of 40g of the warm water (greater than 40°C) into a separate Styrofoam cup and record its temperature in data table 1. 3. As soon as you have recorded the initial temperatures for both samples, pour the cold water into the warm water. 4. Place the lid with the thermometer probe over the calorimeter as pictured below. 5. Gently swirl the mixture in a circular motion. 6. When the temperature of the sample remains constant for several seconds, record it in Data Table 1. Part 2: Varying the mass ratio. 1. Trial 1: Repeat the same procedure from part 1 using 60 grams of warm water and 30 grams of cool water. Record the initial and final temperatures in Data Table 2. 2. Repeat the same procedure from part 1 using 80 grams of warm water and 20 grams of cool water. Record the data in Data Table 2. 3. Repeat the same procedure one more time using 60 grams of warm water and 12 grams of cool water. Record the data in Data Table 2. 1 Physical Science Institute Summer 2013 DATA Data Table 1 Part One Mass Initial Temperature Final Temperature Cold Water Warm Water Data Table 2 Part 2 Mass of Warm Water Initial Temperature (Warm water) Mass of Cold Water 1 60 grams 30 grams 2 80 grams 20 grams 3 60 grams 12 grams Initial Temperature (Cold Water) Final Temperature Decrease in Temperature (Warm water) Increase in Temperature (Cold Water) CALCULATIONS & RESULTS Calculate the increase and decrease in temperatures and record in Data Table 2. PART 1: 1. What was the temperature increase of the cold water? 2. What was the temperature decrease of the warm water? 3. What is the ratio of the warm water temperature decrease to the cold water temperature increase? Share this on the data table on the board. 4. How does this compare with the group? 2 Physical Science Institute Summer 2013 PART 2: 1. Calculate the ratio of the warm water to the cold water. Record in the table below. 2. State the reciprocal of the ratio of masses. Record in the table below. 3. Calculate the decimal equivalent of reciprocal of ratio of masses. Record in the table below. 4. Calculate the ratio of the temperature changes (decrease/increase). Record in the table below. Trial Ratio of masses (warm/cold) Reciprocal of masses Decimal equivalent of reciprocal 1 2 ½ 0.5 Ratio of temperature changes (decrease/increase) 2 3 Which of the two masses underwent the larger temperature change (the larger or smaller mass)? How does the decimal equivalent of the reciprocal of the masses compare to the ratio of temperature changes? Is there a relationship between the warm water mass and temperature decrease, and the cold water mass and its subsequent temperature increase? Represent this relationship as a mathematical equation. We can now use this product to define the change in thermal energy. With this definition, the increase in thermal energy of the cool water equals the decrease in thermal energy of the warm water. In order to define this as an amount of energy, a unit of energy must be chosen. In this case, the unit is: (g)(Δ⁰C); this is also the definition of the calorie, the standard unit of energy used on food labels. 3