Element and Compounds
advertisement
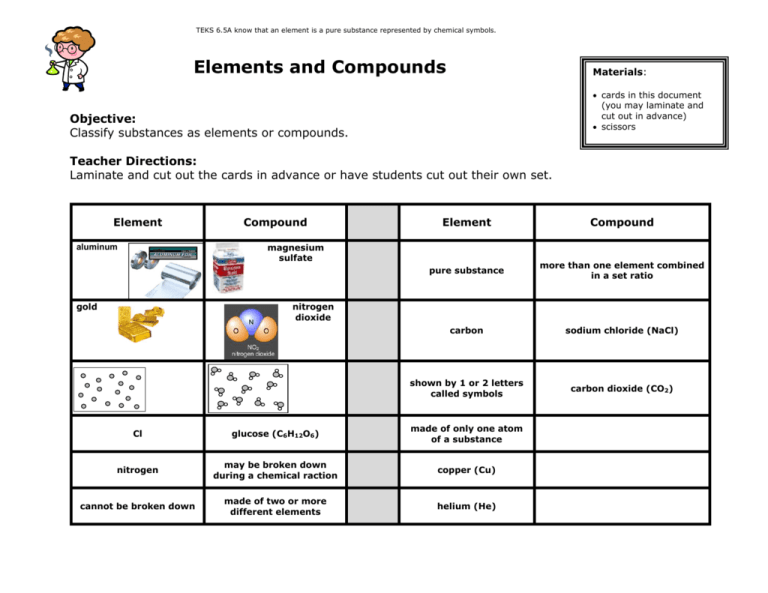
TEKS 6.5A know that an element is a pure substance represented by chemical symbols. Elements and Compounds Materials: cards in this document (you may laminate and cut out in advance) scissors Objective: Classify substances as elements or compounds. Teacher Directions: Laminate and cut out the cards in advance or have students cut out their own set. Element Compound Element Compound pure substance more than one element combined in a set ratio carbon sodium chloride (NaCl) shown by 1 or 2 letters called symbols carbon dioxide (CO2) magnesium sulfate aluminum gold nitrogen dioxide Cl glucose (C6H12O6) made of only one atom of a substance nitrogen may be broken down during a chemical raction copper (Cu) cannot be broken down made of two or more different elements helium (He) TEKS 6.5A know that an element is a pure substance represented by chemical symbols. Elements and Compounds Objective: Classify substances as elements or compounds. Directions: Fold a piece of paper in half. Label one half “element” and the other half “compound”. Cut out the cards. Classify the cards on your paper as elements or compounds. Once you have classified the cards, fill in the table below. Then Answer the questions. Element Compound Element Compound F: Read the following information on elements, compounds and mixtures. Fill in the blanks where necessary. Elements: A pure substance containing only one kind of ____________. An element is always uniform all the way through (homogeneous). An element _____________ be separated into simpler materials (except during nuclear reactions). Over 100 existing elements are listed and classified on the ____________________. Compounds: A pure substance containing two or more kinds of _______________. The atoms are _________________ combined in some way. Often times (but not always) they come together to form groups of atoms called molecules. A compound is always homogeneous (uniform). Compounds ___________________ be separated by physical means. Separating a compound requires a chemical reaction. The properties of a compound are usually different than the properties of the elements it contains. Mixtures: Two or more ________________ or _________________ NOT chemically combined. No reaction between substances. Mixtures can be uniform (called ________________________) and are known as solutions. Mixtures can also be non-uniform (called ________________________). Mixtures can be separated into their components by chemical or physical means. The properties of a mixture are similar to the properties of its components. gold magnesium sulfate helium (He) carbon dioxide (CO2) copper (Cu) sodium chloride (NaCl) glucose (C6H12O6) Cl nitrogen may be broken down during a chemical reaction pure substance made of only one atom of a substance more than one element combined in a set ratio cannot be broken down made of two or shown by 1 or 2 more different letters called elements symbols carbon











