Lesson 16: filled in
advertisement

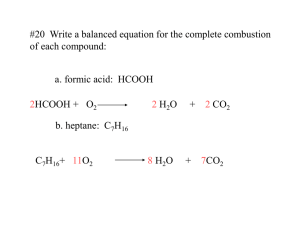
SNC2D1 Date:__________________ Name:_____________________ L16: 6.9 Combustion Reactions and 6.10 Corrosion Combustion: a chemical reaction between a fuel and oxygen (burning of fuel) that produces an oxide and energy. Fuel + oxygen oxides + energy Combustion is a common and useful chemical reaction for our society (burning of fossil fuel). Oxygen is required for a fuel to burn. It is combustion that gives us heat energy for our homes, electricity, and allows us to run our cars. Combustion of Hydrocarbons The most common fuels that we burn are hydrocarbons or fuels made of hydrogen and carbon like natural gas (CH4), gasoline (C8H18), and even candles (C25H52). In each case we need oxygen and produce carbon dioxide and water vapour as well as energy, which we use to run our car, keep us warm or give us light. Word Equation: Methane + Oxygen Carbon dioxide + Water + energy Skeleton Equation: CH4 + O2 CO2 + H2O + energy Word Equation: Skeleton Equation: Gasoline C8H18 + Oxygen + O2 Word Equation: Skeleton Equation: Candle Wax + Oxygen C25H52 + O2 Carbon dioxide + Water + energy CO2 + H2O + energy Carbon dioxide + Water + energy CO2 + H2O + energy In general, the chemical equation for complete combustion of a hydrocarbon is: Hydrocarbon + Oxygen Carbon Dioxide CxHy + O2 CO2 + water + energy + H2O + energy The above combustions are called complete combustions because the fuel is completely burned up. A bright blue color usually indicates that the combustion is complete. Sometimes the fossil fuel or hydrocarbon does not burn completely and this is called incomplete combustion. A bright yellow-orange flickering flame often indicates incomplete combustion. Candles will often burn incompletely producing extra products such as carbon monoxide gas (poisonous) and soot/carbon. (Soot is made up of carbon particles) In general, the chemical equation for incomplete combustion of a hydrocarbon is: Hydrocarbon + Oxygen carbon Dioxide + water + carbon monoxide + carbon + energy CxHy + O2 CO2 + H2 O + CO + C + energy For example: Skeleton Equation: C25H52 + O2 CO2 + H2O + energy + C + CO Other Combustion Reactions Other substance (including elements) can undergo combustion reactions. In general: element + oxygen oxide + energy For example: 2Mg (s) + O2 2MgO (s) + energy 2H2 (g) + O2 2 H2O (g) + energy P4 (s) + 5O2 P4O10 (g) + energy SNC2D1 Corrosion: is the breakdown of a metal resulting from chemical reaction with its environment. chemical properties Examples of corrosion: 1. silver tarnishes over time due to contact with sulfur compounds in the air. 2. Rusting of iron (or metals containing iron like steel). The main factors that cause rusting include exposure to oxygen and water (salt speeds up the corrosion once it has started). 3. Corrosion of some metals (such as aluminum, zinc and copper) causes a tough protective layer to form that protects the metal and prevents further corrosion. To prevent corrosion: 1. The metal can be covered with a protective coating such as rust-inhibiting paint, chrome or plastic. This works until the coating is chipped or scratched. 2. Galvanizing steel is the process of coating it with a thin layer of zinc. Zinc corrodes before iron or steel and forms a protective oxide layer that sticks to the steel. This protection stays intact even if there are scratches much better than paint. 3. Use materials that do not corrode. For example, plastic car bumpers instead of steel; also, surgical steel contains enough chromium so that the medical tools and implants never corrode. In class work: 6.9 Read p. 248-251, complete #2;3 6.10 Read p.252-254; Homework: Start Chapter 6 Review p. 258 #1,3-5, 7-11; p.260 #1-13, 15, 17a, 18, 20