Supplementary Information: Catalyst Activity Test: The schematic of
advertisement

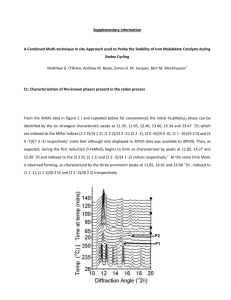
Supplementary Information: Catalyst Activity Test: The schematic of the experimental set-up is shown in Fig. S1. A tubular flow reactor was used to carry out the complete oxidation of cyclohexane in air at atmospheric pressure. The desired inlet concentration of cyclohexane was obtained by introducing an air stream at a constant flow rate to an isolated bubbling saturator filled by liquid cyclohexane which was kept in a refrigeration unit to hold the saturator at a constant temperature. The concentration of cyclohexane in the air flow was calculated by the vapor pressure of cyclohexane at the saturator temperature. A thermocouple was located at the center of the packed reactor to record the oxidation temperature. The feed flow rate was set by rotameters. 1 g of the supported catalyst was placed in the horizontal quartz tubular reactor that was 10 cm in length and 12 mm internal diameter. Supported cobalt oxide catalyst was packed with glass beads into the middle section of the reactor in between glass wool to prevent flow channeling. The reactor was heated to the reaction temperature in the range of (150 to 300oC) and maintained at that temperature using a PID controller. The concentration of cyclohexane in the feed was adjusted in range of 5000 to 20000 ppm. The reactor exit was analyzed using gas chromatography-mass spectroscopy (GCMass, Agilent model) system that was equipped with 5975C mass detector and HP-5MS stainless steel column with 30 m, at regular time intervals from the start of each run. Typical GC-MS for products from the start of a run are seen in Fig. S2. No intermediate oxidation products, carbon monoxide, hydrogen, cylohexene or cyclohexanol were detected by GC-MS, and the products included carbon dioxide, water, and unreacted cyclohexane. To investigate the kinetic behavior of complete oxidation of cyclohexane over HDP catalyst, several experiments were performed to determine the appropriate conditions (catalyst particles size and feed flow rate) to eliminate both internal and external mass transfer resistances. All kinetic experiments were performed with particle size below Mesh number 40 using a feed flow rate of 166 ml/min. Optimization Strategies for Estimation of Kinetic Parameters Fig. S3 represents the GA procedure used in this study that can avoid the trapping in the local optimum. Matlab software version 7.8 was used with appropriate specified parameters included generation size (1000), population size (30), elitecount, crossover fraction (0.9), crossover, and mutation functions. Isothermal plug flow with constant fluid velocity and negligible pressure drop was considered as a simple reactor model. Oxidation Resistance of Almond Shell Based Activated Carbon One of the important properties of activated carbon as a support in the oxidation process is its resistance to oxidation. Figure S4 shows the TGA (Thermogravimetric Analysis) results for almond based activated carbon indicating no oxidation below 450 C. The carbonization of almond shell under water vapor at 800 C had increased the oxidation resistance. Almond activated carbon could therefore be utilized as a support for oxidation of cyclohexane below 400oC and it is stable during BET pretreatment at 300 C. The oxidation resistance of the support was evaluated by thermogravimetric analysis (TGA) using a TGA-25 apparatus (Mettler-Toledo, Switzerland) by heating the sample up to 1000 C under an air flow of 55 cm3/min (STP) and a heating rate of 10 C/min. Catalyst Preparation Methods Almond shells were obtained from Iranian local source as an agricultural solid waste. Powder activated carbon was produced by drying the original almond shells at 120 C for 24 h. The dried shells were grinded then carbonized and activated under water vapor at 800 C in a tubular furnace. Prepared activated carbon was washed with distilled water several times to remove all undesirable materials and was then dried in oven at 110 C overnight. Supported cobalt oxide catalysts with 8wt. % metal loading were prepared by the following two methods: 1) heterogeneous deposition-precipitation (HDP) and 2) combined impregnation and depositionprecipitation (IMP-DP). In the HDP method, powder almond activated carbon (below mesh No.40) was placed in a cobalt nitrate solution (Co(NO3)26H2O) (0.04 M) with heating at 70-80 C and stirring for about 8 h. Cobalt ions were precipitated by addition of NaOH solution (6 wt. %) until reaching a basic solution of pH = 10-11. In the second method, activated carbon was impregnated with cobalt solution at room temperature for about 3 h. The impregnated solution was then heated to reach a temperature in the range of 70-80 C. Heterogeneous DP was then carried out after the impregnation step for 4 h using NaOH. The catalysts prepared by each method were then washed to reach pH = 7 and dried at 110 C for 6 h. The dried samples were calcined for 3 h under N2 at 500 C. Supported cobalt oxide catalysts over almond activated carbon were named IMP-DPCo8/AC500 and HDPCo8/AC500 for IMP-DP and HDP method, respectively. Details and the flow charts (Figs. S5 and S6) for the catalyst preparation techniques are presented in the supplementary information file. Figure Captions: Figure S1: Schematic of the experimental set-up Figure S2: GC-Mass of products from catalytic oxidation of cyclohexane at (a) 10 min and (b) 60 min from start-of run Figure S3: Genetic Algorithm flowchart for kinetic parameter estimation Figure S4: TGA result for almond shell based activated carbon (heating the sample up to 1000 C under an air flow of 55 cm3/min (STP) and a heating rate of 10 C/min). Figure S5: Flow chart of heterogeneous deposition-precipitation method. Figure S6: Flow chart of combined impregnation and deposition-precipitation method. Figure S1: Figure S2: Figure S3: Start Optimization Produce Initial Population (population size = 30) Evaluate Population Crossover (0.9) Mutation No Objective criteria Generation size = 1000 Yes End Figure S4: 100 Weight (%) 80 60 40 20 0 0 200 400 600 Temperature (oC) Figure S5: 800 1000 Figure S6: