Redox Reactions Worksheet: AP Chemistry Practice
advertisement
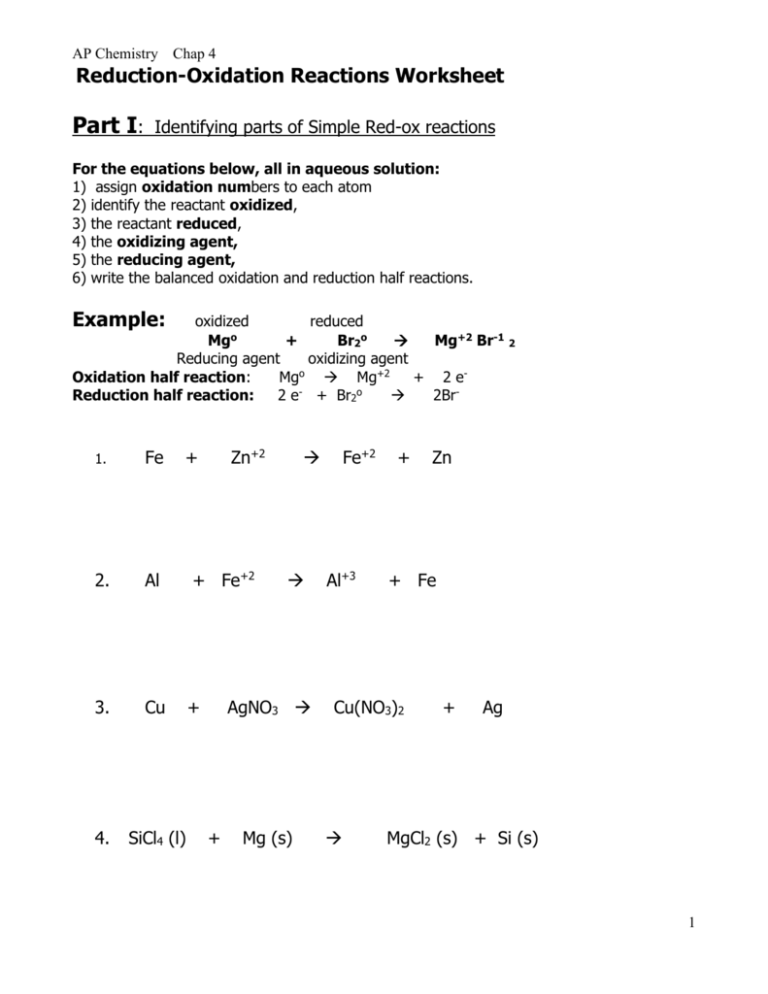
AP Chemistry Chap 4 Reduction-Oxidation Reactions Worksheet Part I: Identifying parts of Simple Red-ox reactions For the equations below, all in aqueous solution: 1) assign oxidation numbers to each atom 2) identify the reactant oxidized, 3) the reactant reduced, 4) the oxidizing agent, 5) the reducing agent, 6) write the balanced oxidation and reduction half reactions. Example: oxidized reduced Mgo + Br2o Mg+2 Br-1 2 Reducing agent oxidizing agent o Oxidation half reaction: Mg Mg+2 + 2 eo Reduction half reaction: 2 e + Br2 2Br- 1. Fe + Zn+2 2. Al + Fe+2 3. Cu + 4. SiCl4 (l) AgNO3 + Mg (s) Fe+2 Al+3 + + Fe Cu(NO3)2 Zn + Ag MgCl2 (s) + Si (s) 1 AP Chemistry Chap 4 Reduction-Oxidation Reactions Worksheet Part II: Balancing Reduction-Oxidation reactions : 2 methods Method 1: a. b. c. d. e. f. Oxidation state method: Write the unbalanced equation. Determine the oxidation states of all atoms in the reactants and products. Show electrons gained and lost using “tie lines.” Use coefficients to equalize the electrons gained and lost. Balance the rest of the equation by inspection. Add appropriate states. Method 2: Half Reaction method: A. In Acid Solution: 1.Write separate reduction, oxidation reactions. 2. For each half-reaction: Major, O,H,e- remember it by “Major Hydroxide” Major species: Balance elements (except H, O) Balance O (oxygen) using H2O Balance H (hydrogen) using H+ Balance charge using electrons (-) 3. If necessary, multiply by integer to equalize electron count. 4. Add half-reactions. 5. Check that elements and charges are balanced. B. In Basic Solution 1. Balance as in acid solution (above). - 2. Then, Add OH to both sides, equal to the Number of H+ ions + 3. Form water by combining H , OH . Cancel from both sides H2O’s as needed. 4. Check elements and charges for balance. Problems: I. Balance using the Oxidation State Method (see your text for example problems) 1. Cr(s) + Pb+2(aq) Cr+3 (aq) + Pb (s) 2. C2H6 (g) + O2 (g) CO2 (g) + H2O (g) 2 AP Chemistry Chap 4 Reduction-Oxidation Reactions Worksheet II. Balance the equations below using the half reaction method. Notice if it is in acid or base solution. 3. NH3 (g) + O2(g) 4. NO (g) + H2O (g) in acid MnO2 (s) + H2C2O4 (aq) + H2SO4 (aq) MnSO4 (aq) + CO2 (g) + H2O (l) in base 3











