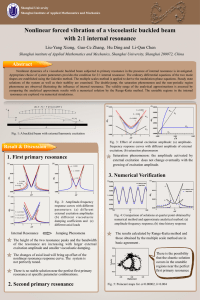
Answers - Chemistry Courses: About
advertisement

Resonance Concepts Major concepts Resonance is not a real phenomenon; it is a tool to more closely describe the reality of molecular bonding Using the arrow formalism, one can draw a series of resonance structures Resonance can be used to explain charge distribution over a molecule A resonance hybrid of a molecule may be drawn based on major and minor resonance contributors An understanding of structural charge distribution is essential to understanding the stability of a structure. Vocabulary Resonance hybrid Charge distribution Arrow formalism Students should be able to: Recognize the difference between resonance structures and constitutional isomers Explain why resonance is a tool, not a phenomenon Recognize major and minor resonance contributors Use resonance structures to draw a resonance hybrid Describe the charge distribution of a molecule using resonance Draw alternate resonance structures for a Lewis structure, given arrows Daily Problems 1. Fill in all hydrogens and lone pairs explicitly in these resonance structures. Notice how, although some bonds and lone pairs are in different places, the atoms are still bonded in the same connectivity. δ- δ- δ+ δ+ δ- δMajor due to neg. charge on the more electronegative atom δ+ + δ δMajor because every Major because there is atom a complete octet nohas charge δ+ 2. In each of the sets of resonance structures in problem 1, draw an arrow formalism that helps get to the next structure. 3. Label each of the structures in problem 1 as major or minor resonance contributor, and explain why. (Don’t worry about parts A and B.) 4. Draw a resonance hybrid for each of the molecules in problem 1. 5. Given the arrows, draw a resonance structure. 6. Circle all structures which are resonance structures of Compound A. Explain why the other structures are not resonance structures. (Hint: Drawing in implicit H’s and lone pairs is helpful.) A C B D B and D are resonance structures of compound A. A and C are NOT resonance structures of A. Drawing in the implicit hydrogens will show you that they do not have the same molecular formulaand there is a charge missing when the double bond gets “moved”. Cumulative Problems 7. A beginning R340 student attempted to draw these resonance structures based on these arrows. They are wrong. Explain what is wrong with the resonance structure and/or the arrows. You cannot give electrons to a carbon atom that already has a full octet. The way the arrow is drawn implies that electrons are being taken away from oxygen, yet the resonance structure has a negative charge on the oxygen. The arrow pushing is backwards. These are two different compounds. There is an extra hydrogen on carbon 2. 8. Using resonance and/or induction principles, indicate which of the carbon atoms in these molecules are Lewis acidic and which are Lewis basic. (At this point, you have been given arrows to help you draw resonance structures. After next lecture, they won’t be given.) LB LA LA LB LB LB LA LA LA 9. Often, the charge distribution in a molecule shown by resonance stabilizes the molecule, but sometimes it destabilizes the molecule. A. Consider these two ions. The carbon attached to chlorine has what partial charge in each case? Fill in the charge. δ+ δ+ B. Based on the resonance arrows given, draw a second resonance structure. C. Draw the resonance hybrid for each ion based on the resonance structures from part B. Then indicate the partial charge due to induction from part A on the structure. Compound A δ- δ- Compound B δ+ δ+ D. Which compound is stabilized by resonance? Which is destabilized? Compound A is stabilized by resonance. The partial negative and partial positive (on the carbon beside the chlorine) would attract therefore stabilizing the compound. Compound B is destabilized because the partial positive charges would repel each other. Extension problems 10. Vitamin C is drawn below, along with one resonance structure. Draw one more resonance structure that puts the negative charge on a different oxygen atom.