Virtual Building Molecules Lab
advertisement

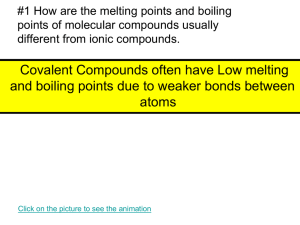
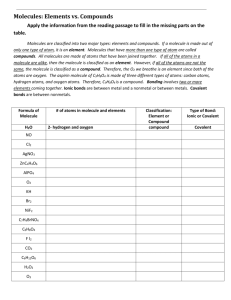
Name: ________________________________________________ Period: _________ Building Molecules Virtual Activity Introduction: Scientists use a system to classify matter based on its chemical structure. Elements are substances in which all the atoms are alike. Compounds are made up of two or more elements that are chemically combined. Many common materials are compounds. For instance, water is a compound that is made up of hydrogen and oxygen atoms. Many compounds are made up of molecules. A molecule is two or more atoms chemically bonded to each other. It is the smallest particle of a substance that has all the properties of that substance. A molecule of water contains 2 hydrogen atoms chemically bonded to 1 oxygen atom. The chemical makeup of water can be expressed in a chemical formula: H2O. The number 2 placed to the lower right of the chemical symbol H (for hydrogen) is called a subscript. It indicates that there are two atoms of hydrogen in a molecule of water. When a chemical symbol has no subscript, it means there is only one atom of that element in the molecule. There is no number to the right of the chemical symbol O (for oxygen); therefore, a molecule of water contains 1 atom of oxygen. Many of the physical and chemical properties of a molecule are determined by its shape. The 3-dimensional shapes of molecules can be pictured by using molecular models. To make a model of a molecule, you need to know two things: its chemical formula and how its atoms fit together. In chemistry, different types of atoms bond with other atoms in specific ways. For instance, hydrogen will tend to form one bond with another atom, and oxygen will tend to form two bonds. In some cases, multiple bonds form between atoms. A double bond counts as the same as two bonds, and a triple bond counts the same as three bonds. In this virtual lab, you will build molecular models of various elements and compounds, given their chemical formulas. For some molecules, there is more than one way to build a model. Objectives: Explain what a molecule is. Explain what a compound is. Construct a model of a molecule based on its chemical formula. Procedure: 1. Go to the website: http://www.glencoe.com/sites/common_assets/science/virtual_labs/E02/E02.swf or Google “Glencoe Virtual Molecule Lab” and click on the first link. 2. Click the Information button to learn about the structure of molecules. 3. Choose a molecule from the Molecule pull-down menu. 4. Drag atoms and bonds from the Atoms and Bonds clone pads to build a structural model of the molecule you chose. 5. Click the Check button to check your model. 6. If your model is correct, click the 3-D Model Button to see a three-dimensional model of the molecule. 7. Record your findings in the table. Results: Molecule Name Ammonia Carbon dioxide Ethyne Hydrogen Hydrogen peroxide Methane Nitrogen Oxygen Propane Water Chemical Formula Drawing of Correct Molecule Model Number of Hydrogen Atoms Number of Oxygen Atoms Number of Nitrogen Atoms Number of Carbon Atoms Analysis Questions: 1. What is an element? 2. What is a compound? 3. What is a molecule? 4. What is the difference between a molecular formula and a molecular model? 5. Describe the process you used to build a model. What did you do first? Second? 6. Did you create a model that looked different from the model shown when you clicked the 3-D Model? If so, how was it different?