Honors Chemistry WS 7.1 Solubility What is a solution? Give specific
advertisement

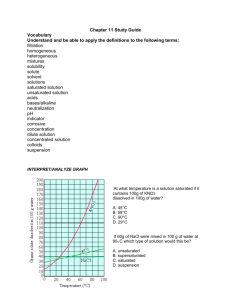
Honors Chemistry WS 7.1 Solubility 1. What is a solution? Give specific examples of three different types of solutions: solid dissolved in a liquid, gas dissolved in a liquid, and gas dissolved in a gas. 2. Discuss how an ionic solute such as NaCl dissolves in water. How are the strong ionic bonds in the solid overcome to permit the solid to dissolve? 3. Explain how a polar covalent molecule such as a simple sugar (C6H12O6) can dissolve in water? 4. What determines whether a solute will be soluble in a given solvent? 5. When KNO3 is added to water, the energy required to separate the solute particles is +250kJ/mol, the energy required to separate the solvent molecules is +120 kJ/mol and the energy released when the solute and solvent particles form bonds is -400 kJ/mol. a) Calculate the ∆𝐻𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 b) Will the solution warm up or cool off? Explain. 6. Predict (circle) which solvent will dissolve more of the given solute: a) NaCl in methanol (CH3OH) or in 1-propanol (CH3CH2CH2OH) b) Ethylene glycol (HOCH2CH2OH) in hexane (C6H14) or in water. c) Diethyl ether (CH3CH2OCH2CH3) in water or in ethanol (CH3CH2OH) 7. Will sugar, C6H12O6 a polar covalent molecule, dissolve in nail polish remover, a nonpolar solvent? Explain. 8. Will oil, a long carbon chain hydrocarbon (nonpolar) dissolve in water? Explain. 9. Hydrogen chloride (HCl) gas is much more soluble than propane (C3H8) in water, even though HCl has a lower boiling point. Explain. 10. What is the strongest type of intermolecular force between solute and solvent in each solution? a) CH3OH(l) in CCl4(l) __________________________ b) CH3Cl(g) in CH3OCH3(g) __________________________ c) NaCl(s) in H2O(l) __________________________ 1 11. How does the solubility of a gas in liquid depend on temperature? How does this temperature dependence affect the amount of oxygen available to aquatic animals? 12. How does the solubility of a gas depend on pressure? How does this pressure dependence account for the bubbling that occurs upon opening a can of soda? 13. Fill in the chart below with either increases, decreases, or remains the same for changing the following factors on the SOLUBILITY of the solute in water: Solute CO2(g) NaCl(s) increasing temp decreasing temp increasing pressure decreasing pressure 14. What is the concentration of CO2 (k = 3.1 x 10-2 mol/L•atm) that is bottled with a partial pressure of 4.0 atm? 15. What is the concentration of N2 (k = 6.8 x 10-4 mol/L-atm) in a diver's blood if he breaths air at 2.5 atm that is 78 % N 2. 16. A solution is made up of 123 g NaOH and 289 g water. The total volume is 300.0 mL. Determine mole NaOH mole H2O mass % mole fraction 2 molarity PPM 17. Using the solubility graph, determine if the following solutions are saturated, unsaturated or supersaturated. If they are anything but saturated, list two things you can do to make them saturated (include numbers). Solution (in 100g H2O) Sat, Unsat, Supersat +/- how many °C to make saturated? +/- how many g to make saturated? 40 g of KClO3 at 50oC 90 g NaNO3 at 10oC 70 g KNO3 at 60oC 80 g Pb(NO3)2 at 40oC 22. A 100.5 mL intravenous (IV) solution contains 5.10 g of glucose (C6H12O6). What is the molarity of this solution? 23. What is the molarity of a bleach solution containing 9.5 g of NaOCl per liter of bleach? 3 24. Cow’s milk typically contains 4.5% by mass of lactose, C12H22O11. Calculate the mass of lactose present in 175 g of milk. 25. A solution is prepared by mixing 1.00 g of ethanol, C2H5OH, with 100.0 g of water. Calculate the mass percent of ethanol in this solution. 26. Dilution problem. What volume of 16 M sulfuric acid must be used to prepare 1.5L of a 0.10 M H2SO4 solution? 27. PPM problem. If .5mL of blood are added to 10.0 L of water what is the concentration in PPM? 28. Dilution problem. How many milliliters of a 5.0 M H2SO4 stock solution would you need to prepare 100.0mL of 0.25 M H2SO4? 29. What is the percent by volume of ethanol in a solution that contains 35 mL of ethanol dissolved in 115 mL of water? 30. Calculate the molarity of 1.60L of a solution containing 1.55 g of dissolved KBr. 18. How would you prepare 250.0 mL of a 0.127 M Ca(OH)2 a. from powder Ca(OH)2? b. from 1.00 M Ca(OH)2? 4 5