Volume of air (mL)
advertisement
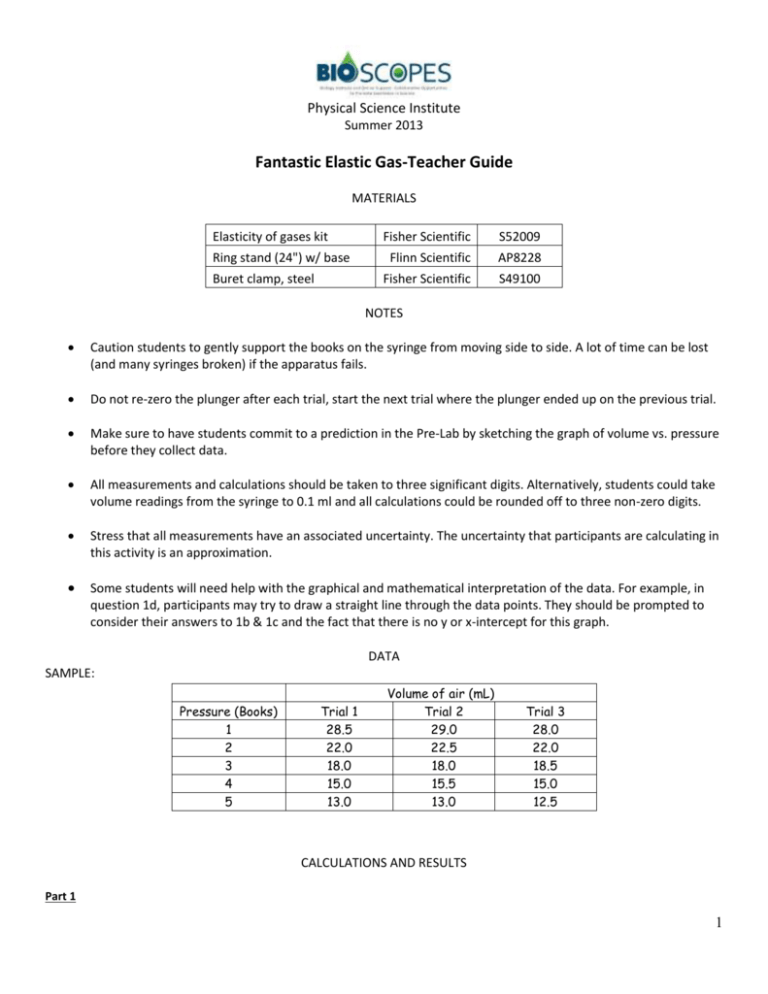
Physical Science Institute Summer 2013 Fantastic Elastic Gas-Teacher Guide MATERIALS Elasticity of gases kit Ring stand (24") w/ base Buret clamp, steel Fisher Scientific S52009 Flinn Scientific AP8228 Fisher Scientific S49100 NOTES Caution students to gently support the books on the syringe from moving side to side. A lot of time can be lost (and many syringes broken) if the apparatus fails. Do not re-zero the plunger after each trial, start the next trial where the plunger ended up on the previous trial. Make sure to have students commit to a prediction in the Pre-Lab by sketching the graph of volume vs. pressure before they collect data. All measurements and calculations should be taken to three significant digits. Alternatively, students could take volume readings from the syringe to 0.1 ml and all calculations could be rounded off to three non-zero digits. Stress that all measurements have an associated uncertainty. The uncertainty that participants are calculating in this activity is an approximation. Some students will need help with the graphical and mathematical interpretation of the data. For example, in question 1d, participants may try to draw a straight line through the data points. They should be prompted to consider their answers to 1b & 1c and the fact that there is no y or x-intercept for this graph. DATA SAMPLE: Pressure (Books) 1 2 3 4 5 Trial 1 28.5 22.0 18.0 15.0 13.0 Volume of air (mL) Trial 2 29.0 22.5 18.0 15.5 13.0 Trial 3 28.0 22.0 18.5 15.0 12.5 CALCULATIONS AND RESULTS Part 1 1 Physical Science Institute Summer 2013 1. Answers will vary 2.-5. Table 4.1 P (books) 1 2 3 4 5 Avg. Vol. (mL) Uncertainty in Avg. Volume (mL) Highest volume (mL) Lowest volume (mL) 28.5 + 0.5 29.0 28.0 22.2 + 0.3 22.5 21.9 18.2 + 0.3 18.5 18.2 15.2 + 0.3 15.5 14.9 12.8 + 0.3 13.1 12.5 Question 1: a. The volume of the air trapped in the syringe decreased. b. To get the volume to zero one would need an infinite number of books (pressure). c. The resulting line is a smooth curve with asymptotes between the x & y axis (see example below). 2 Physical Science Institute Summer 2013 Part 2 7. & 8. Table 4.2 P (books) Avg. 1/V (mL-1) Low 1/V (mL-1) High 1/V (mL-1) 1 0.0351 0.0345 0.0357 2 0.0450 0.0444 0.0457 3 0.0549 0.0541 0.0559 4 0.0658 0.0645 0.0672 5 0.0781 0.0763 0.0800 3 Physical Science Institute Summer 2013 9. Atmospheric pressure = 2.2 books Question 2: a. The y-intercept for the sample data is -2.2 books b. When there were no books, the total pressure does not equal zero (Ptotal ≠ 0 books). There is another pressure on the plunger that we neglected. c. Atmospheric pressure on the plunger, in books. d. V approaches infinity (∞). EXTENSION Using the slope-intercept form for a straight line equation our data yields: 4 Physical Science Institute Summer 2013 P(books on plunger) = (93 book*mL)(1/V) + (-2.2 books) POSTLAB DISCUSSION Once students have calculated their value for the atmospheric pressure in books, have them determine the TOTAL pressure (P(books) + Patm (books) = TOTAL pressure). They can then multiply TOTAL pressure times the average volume from Part 1. They should see that: TOTAL pressure x avg. volume = constant (Boyle’s Law). Atmospheric Pressure (books) Total Pressure (books) Total Pressure x avg. volume (book mL) 2.2 3.2 91 2.2 4.2 93 2.2 5.2 95 2.2 6.2 94 2.2 7.2 92 5