histo 698 to 726 [10-4
advertisement

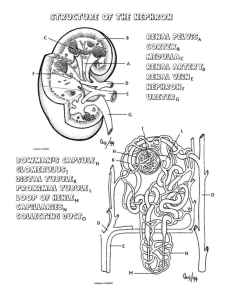
histo 698 to 726 Kidneys Conserve water, essential electrolytes, and metabolites Remove waste products of metabolism Essential in maintaining acid-base balance by excreting hydrogen ions when bodily fluids become too acidic or excreting bicarbonates when bodily fluids become too basic Receive about 25% of cardiac output Creatinine gets excreted in the urine Kidneys and Vitamin D Vitamin D3 (cholecalciferol) produced in skin from 7-dehydrocholesterol by action of UV light o 30 min to 2 hours of sunshine exposure per day can provide enough vitamin D fo daily body requirements Vitamin D3 is also absorbed by the small intestine in association with chylomicrons In blood, vitamin D3 is bound to vitamin D-binding protein and transported to liver In liver, vitamin D3 is hydroxylated to become 25-OH vitamin D3, which is released into the bloodstream and undergoes a second hydroxylation in proximal tubules of kidney to produce 1,25-dihydroxy vitamin D3 (calcitriol) Hydroxylation in proximal tubules of kidneys regulated indirectly by an increase in plasma Ca2+, which triggers secretion of PTH o Can also be regulated directly by decrease in circulating phosphates, which stimulates activity of 1 αhydroxylase responsible for the conversion Active 1,25-dihydroxy vitamin D3 stimulates intestinal absorption of Ca2+ and phosphate and mobilization of Ca2+ from bones, so it is necessary for normal growth of bones and teeth Vitamin D2 (ergocalciferol) – undergoes the same conversion process and has the same biologic effects Patients with end-stage chronic kidney diseases have inadequate conversion of vitamin D into active metabolites resulting in vitamin D3 deficiency – manifested by impaired bone mineralization and reduced bone density o Increase secondary hyperparathyroidism o Have a diet supplemented with vitamin D3 and calcium Structure of Kidneys Endocrine activities o Synthesis and secretion of erythropoietin (EPO), which acts on bone marrow and regulates RBC formation in response to decreased blood oxygen concentration EPO synthesized by endothelial cells of peritubular capillaries in renal cortex and acts on specific receptors expressed on surface of erhthrocyte progenitor cells (Er-P) in bone marrow Recombinant form of EPO (RhEPO) used for treatment of anemia in patients with end-stage renal disease – also used to treat anemia resulting from bone marrow suppression that develops in AIDS patients undergoing treatment with antiretroviral drugs, such as azidothymidine (AZT) o Synthesis and secretion of renin (enzyme involved in control of BP and blood volume) – produced by juxtaglomerular cells and cleaves circulating angiotensinogen to release angiotensin I o Hydroxylation of 25-OH vitamin D3 to hormonally active 1,25-dihydroxy vitamin D3 – regulated primarily by PTH, which stimulates activity of 1α-hydroxylase and increases production of active hormone Extend from T12-L3, with right kidney slightly lower than left Kidney surface covered by CT capsule that consists of 2 layers o Outer layer of fibroblasts and collagen fibers o Inner layer with cellular component of myofibroblasts Contractility of myofibroblasts aids in resisting volume and pressure variations that can accompany variations in kidney function Capsule passes inward at hilum, where it forms CT covering of sinus and becomes continuous with CT forming walls of calyces and renal pelvis About 90% of blood passing through kidney is in the cortex Cortex consists of renal corpuscles along with convoluted tubules and straight tubules of nephron, collecting tubules, collecting ducts, and extensive vascular supply o Renal corpuscles are spherical structures that constitute the beginning segment of the nephron and contain the glomerulus Medullary rays (of Ferrein) – vertical striations that emanate from the medulla and project into the cortex o Each medullary ray contains straight tubules and collecting ducts o Regions between medullary rays contain renal corpuscles, convoluted tubules, and collecting tubules o Regions between medullary rays – cortical labyrinths o Each nephron and its collecting tubule (which connects to a collecting duct in the medullary ray) form a uriniferous tubule Straight tubules and collecting ducts continue from cortex into medulla, accompanied by vasa recta (capillary network that runs in parallel with various tubules) o Represent vascular part of countercurrent exchange system that regulates concentration of urine Tubules in medulla form pyramids – bases face cortex and apices face renal sinus o Each pyramid is divided into outer medulla (adjacent to cortex) and inner medulla o Outer medulla divided into inner stripe and outer stripe Renal columns (of Bertin) – caps of cortical tissue that lie over pyramids – extend peripherally around lateral portion of pyramid o Contain the same components as cortical tissue but are regarded as part of the medulla Papilla – apical portion of each pyramid that projects into a minor calyx (cup-shaped structure that represents an extension of the renal pelvis) o Area cribrosa – tip of papilla – perforated by openings of collecting ducts Minor calyces are branches of major calyces that are the major divisions of the renal pelvis Kidney Lobes and Lobules Lobe – single medullary pyramid and its associated cortical tissue at base and sides (one half of each adjacent renal column) o Each reflected as a convexity on the outer surface of the organ, but usually disappear after birth Lobules – central medullary ray and surrounding cortical material o Boundaries not obviously demarcated from one another by septa o Medullary ray containing collecting duct for a group of nephrons that drain into that duct constitutes the renal secretory unit o Each renal secretory unit is a lobule Nephron Each kidney has about 2 million nephrons, which are responsible for production of urine and correspond to secretory part of other glands Nephrons and their collecting tubules arise from separate primordial and only later become connected Renal corpuscle – beginning of the nephron and made of the glomerulus and the renal capsule (Bowman’s capsule) o Bowman’s capsule – initial portion of the nephron where blood flowing through glomerular capillaries undergoes filtration to produce glomerular ultrafiltrate Glomerular capillaries supplied by afferent arteriole and is drained by efferent arteriole that then branches, forming a new capillary network to supply kidney tubules o Site where afferent and efferent arterioles penetrate and exit from parietal layer of Bowman’s capsule is vascular pole (opposite urinary pole of renal corpuscle where proximal convoluted tubule begins) Proximal thick segment – proximal convoluted tubule (pars convolute) and proximal straight tubule (pars recta) Thin segment – thin part of loop of Henle Distal thick segment – distal straight tubule (pars recta) and distal convoluted tubule (pars convolute) Distal convoluted tubule connects to collecting tubule, often through a connecting tubule, forming the uriniferous tubule (nephron plus collecting tubule) Beginning from Bowman’s capsule, the sequential parts are o Proximal convoluted tubule – originates from urinary pole of Bowman’s capsule – very tortuous course and then enters medullary ray to continue as proximal straight tubule o Proximal straight tubule – thick descending loop of Henle, descends into medulla o Thin descending limb – continuation of proximal straight tubule within medulla, maks a hairpin turn and returns toward cortex o Thin ascending limb – continuation of thin descending limb after the hairpin turn o Distal straight tubule – thick ascending loop of Henle, continuation of thin ascending limb – ascends through medulla and enters cortex in medullary ray to reach renal corpuscle or origin, then leaves medullary ray and makes contact with vascular pole of its parent renal corpuscle, where the epithelial cells of the tubule adjacent to the afferent arteriole of the glomerulus are modified to form the macula densa o Distal convoluted tubule – less tortuous than proximal convoluted tubule and empties into collecting duct that lies in medullary ray via an arched collecting tubule or a shorter tubule (connecting tubule) Types of Nephrons Subcapsular nephrons (cortical nephrons) – renal corpuscles located in outer part of cortex o Have short loops of Henle, extending only into outer medulla o Hairpin turn occurs in distal straight tubule Juxtamedullary nephrons – about 1/8 of total nephron count o Renal corpuscles occur in proximity to base of medullary pyramid o Long loops of Henle and long ascending thin segments that extend well into inner region of pyramid Intermediate nephrons (midcortical nephrons) – have renal corpuscles in midregion of cortex o Loops of Henle are intermediate in length Collecting Tubules and Ducts Collecting tubules begin in cortical labyrinth as either connecting tubules or arched collecting tubules and proceed to medullary ray, where they join collecting ducts Collecting ducts in cortex called cortical collecting ducts, and when collecting duct reaches medulla, it is a medullary collecting duct Medullary collecting ducts travel to apex of pyramid, where they merge into larger collecting ducts (papillary ducts or ducts of Bellini) that open into minor calyx Area in papilla that contains openings of collecting ducts is area cribrosa Filtration Apparatus in Kidney Renal corpuscle – glomerular capillary tuft and surrounding visceral and parietal epithelial layers of Bowman’s capsule Glomerular filtration barrier – enclosed by parietal layer of Bowman’s capsule, consisting of o Endothelium of glomerular capillaries – possess numerous fenestrations that are larger, more numerous, and more irregular in outline than normal fenestrations in other capillaries Has no diaphragm over these fenestrations Possess large number of aquaporin-1 (AQP-1) water channels that allow fast movement of water through epithelium o Glomerular basement membrane (GBM) – thick basal lamina that is joint product of endothelium and podocytes (cells of visceral layer of Bowman’s capsule) Composed of network of type IV collagen, laminin, nidogen, entactin, proteoglycans such as agrin and perlecan, and multiadhesive glycoproteins Mutation in gene encoding for α5 chain of type IV collagen – Alport’s syndrome (hereditary glomerulonephritis) – manifests with hematuria, proteinuria, and progressive renal failure – GBM irregularly thickened with laminated lamina densa and fails to serve as effective filtration barrier o Visceral layer of Bowman’s capsule – contains podocytes (visceral epithelial cells) that extend processes around the glomerular capillaries Podocytes arise during embryology from one of blind ends of developing nephron through invagination of end of tubule to form double-layered epithelial cup – cup eventually closes to form spherical structure containing the glomerulus As they differentiate, podocytes extend processes around capillaries and develop numerous secondary processes (pedicels or foot processes) that interdigitate with foot processes of neighboring podocytes Filtration slits – spaces between foot processes – covered by filtration slit diaphragm that spans filtration slit slightly above GBM Filtration slit diaphragm has complex zipper-like sheet configuration o Nephrin molecules emerging from opposite foot processes interact in center of slit, forming a central density with pores on both sides o Filtration slit diaphragm firmly anchored to numerous actin filaments within foot processes of podocytes o Mutations in nephrin gene (NPHS1) associated with congenital nephrotic syndrome (massive proteinuria and edema) Filtration apparatus allows for high filtration rate of water, unrestricted passage of small and middle-sized molecules, and almost total exclusion of serum albumins and other larger proteins o Has 2 layers – endothelium of glomerular capillaries and visceral layer of Bowman’s capsule – these two layers on either side of glomerular basement membrane o Endothelial surface layer of glomerular capillaries – carb-rich meshwork attached to luminal surface of glomerular endothelial cells – contains glycocalyx Plasma proteins adsorbed from blood coat luminal surface of glycocalyx o Subpodocyte space – narrow space between foot processes with their filtration slit diaphragms on one side and a cell body of the podocytes on the other side GBM – contains type IV and XVIII collagens, sialoglycoproteins, and other noncollagenous glycoproteins, as well as proteoglycans and glycosaminoglycans o Lamina rara externa – adjacent to podocytes processes – particularly rich in polyanions that specifically impede passage of negatively charged molecules o Lamina rara interna – adjacent to capillary endothelium – similar to lamina rara externa o Lamina densa – overlapping portion of the two basal laminae, sandwiched between laminae rarae – contains type IV collagen, which is organized into a network that acts as a physical filter Type XVIII collagen, perlecan, and agrin responsible for bulk of anionic charges found in GBM Laminin and other proteins present in laminae rara interna and externa involved in attachment of endothelial cells and podocytes to GBM Proteins that do get through GBM are reabsorbed by endocytosis in proximal convoluted tubule Albuminuria and hematuria are caused by significant reduction in number of anionic sites in GBM, especially lamina rara externa Filters of filtration unit of nephron prevented from clogging by negative charges of GAG’s in GBM, negative charges of podocytes PM, and phagocytic function of mesangial cells in renal corpuscle Glomerular filtration barrier is an active structure that can remodel itself and modify its own permeability as needed Parietal layer of Bowman’s capsule contains parietal epithelial cells and forms a simple squamous epithelium o At urinary pole of renal corpuscle, parietal layer continuous with cuboidal epithelium of proximal convoluted tubule o Proliferation of parietal epithelial cells is typical diagnostic feature of glomerulonephritis o Space between visceral and parietal layers of Bowman’s capsule called urinary space (Bowman’s space) and is receptacle for glomerular ultrafiltrate (primary urine) produced by filtration apparatus of renal corpuscle o At urinary pole, urinary space is continuous with lumen of proximal convoluted tubule Mesangium Mesangial cells and their ECM = mesangium Most obvious at vascular stalk of glomerulus and at interstices of adjoining glomerular capillaries Enclosed by GBM Not confined entirely to renal corpuscle – some located outside corpuscle along vascular pole, where they are also called lacis cells and form part of juxtaglomerular apparatus Mesangial cell functions o Remove trapped residues aggregated proteins from GBM and filtration slit diaphragm o Endocytose and process variety of plasma proteins including immune complexes o Main function is maintenance of structure and function of glomerular barrier o Produce components of mesangial matrix that provide support for podocytes in areas where epithelial basement membrane is absent or incomplete o Synthesize and secrete interleukin 1 (IL-1), PGE2, and platelet-derived growth factor (PDGF), which play a central role in response to glomerular injury o Proliferate in certain kidney disease, in which abnormal amounts of protein and protein complexes are trapped in GBM Prominent feature in IgA nephropathy (Berger disease), membranoproliferative glomerulonephritis, lupus nephritis, and diabetic nephropathy Embryologically derived from smooth muscle cell precursors Juxtaglomerular Apparatus Includes macula densa, juxtaglomerular cells, and extraglomerular mesangial cells Macula densa – directly adjacent to afferent and efferent arterioles and adjacent to some extraglomerular mesangial cells at vascular pole of renal corpuscle in terminal portion of distal straight tubule of nephron o Macula densa cells narrower and taller than other distal tubule cells o Nuclei appear crowded to the point of being partially superimposed over each other In same region as macula densa, smooth muscle cells of adjacent afferent arteriole (and sometimes efferent arteriole) modified to contain secretory granules and spherical nuclei – these are juxtaglomerular cells Juxtaglomerular cells activate renin-angiotensin-aldosterone system (RAAS) during low sodium intake or pathological conditions that reduce volume of circulating blood or reduce renal perfusion o Granules of juxtaglomerular cells contain renin, which is synthesized, stored, and released into blood stream from modified smooth muscle cells o In blood, renin catalyzes hydrolysis of circulating angiotensinogen to produce angiotensin I o Angiotensin I converted to active angiotensin II by angiotensin-converting enzyme (ACE) present in endothelial cells of lung capillaries o Angiotensin II stimulates synthesis and release of aldosterone from zona glomerulosa of adrenal gland o Aldosterone acts on collecting ducts to increase reabsorption of sodium and concomitant reabsorption of water, thereby raising blood volume and pressure o Angiotensin II potent vasoconstrictor that has regulatory role in control of renal and systemic vascular resistance Functions as sensor of blood volume and tubular fluid composition o Cells of macula densa monitor Na+ concentration in tubular fluid and regulate both glomerular filtration rate and release of renin from juxtaglomerular cells o Decreased Na+ in distal convoluted tubule stimulates ion-transporting molecules on apical membrane of macula densa cells that include Na+/2Cl-/K+ cotransporters, Na+/H+ exchangers, and pH-regulated and calcium-regulated K+ channels Initiates signaling by releasing various mediators such as ATP, adenosine, NO, and PGE2 (prostaglandins) that act in paracrine manner and signal both underlying juxtaglomerular cells of afferent arteriole to secrete renin and vascular smooth muscle cells to contract o Increase in blood volume sufficient to cause stretching of juxtaglomerular cells in afferent arteriole is same stimulus that closes feedback loop and stops secretion of renin ABMAIG and Goodpasture Syndrome Major building block of GBM is type IV collagen An autoimmune response to noncollagenous NC1 domain of above type IV collagen is responsible for development of anti-GBM antibody-induced glomerulonephritis, characterized by linear deposition of IgG antibodies in GBM Goodpasture syndrome – when anti-GBM antibodies cross-react with alveolar basement membrane in lungs o Clinical feature is rapidly progressive glomerulonephritis and pulmonary hemorrhage due to disruption of air-blood barrier o In response to deposition of IgG in glomerulus, complement system activated and circulating leukocytes elaborate proteases, leading to disruption of GBM and deposition of fibrin, which stimulates proliferation of parietal cells lining Bowman’s capsule and cause influx of monocytes from circulation o Product of these reactions is seen in glomerulus as crescent o Most patients affected have severe crescentic glomerulonephritis with transiently elevated levels of circulating anti-GBM antibodies o Formation of anti-GBM antibodies most likely triggered by viruses, cancers, pharmacologic agents, and chemical compounds in paints, solvents, and dyes o Patients present with both respiratory and urinary problems (SOB, cough, bloody sputum, hematuria, proteinuria, and other kidney failure symptoms) Main therapeutic goal is to remove circulating pathogenic antibodies from blood by plasmapheresis (blood plasma removed from circulation and replaced by fluid, protein, or donated plasma Treatment with immunosuppressive drugs and corticosteroids is beneficial to keep immune system from producing pathogenic autoantibodies Urinalysis Excessive excretion of protein almost always indicates renal disease, but can be caused by extreme exercise or severe dehydration Microscopic examination is looking for RBC’s, WBC’s, mineral crystals, and pathogenic agents such as bacteria or fungi o Often these are enclosed in cylindrical structures called urinary casts o Matrix of urinary cast formed by uromodulin (Tamm-Horsfall protein) that precipitates in lumen of distal convoluted tubules and collecting ducts during disease process RAAS and Hypertension Chronic essential hypertension – most common form of hypertension True cause of RAAS discovered from venom of SA snake shown to be an ACE (angiotensin-converting enzyme) inhibitor in lungs “lesion” in chronic essential hypertension is excessive production of angiotensin II in lung ACE inhibitor drugs do not cause often-dangerous side effects of diuretics and β-blockers that were most commonly used drugs before ACE-inhibitors Kidney Tubule Function As glomerular ultrafiltrate passes through uriniferous and collecting tubules o Certain substances within ultrafiltrate are reabsorbed Partially (water, sodium, bicarbonate) Completely (glucose) o Other substances (creatinine and organic acids and bases) added to ultrafiltrate by secretory activity of tubule cells Loop of Henle and collecting tubules that pass parallel to similarly arranged blood vessels (vasa recta) serve as basis for countercurrent multiplier mechanism instrumental in concentrating urine, making it hyperosmotic Proximal Convoluted Tubule Receives ultrafiltrate from urinary space of Bowman’s capsule Cuboidal cells of proximal convoluted tubule have o Brush border composed of relatively long, closely packed, straight microvilli o Junctional complex consisting of narrow tight junction that seals off intercellular space from lumen of tubule and zonula adherens that maintains adhesion between neighboring cells o Plicae (folds) located on lateral surfaces of cells, which are large flattened processes alternating with similar processes of adjacent cells o Extensive interdigitation of basal processes of adjacent cells o Basal striations consisting of elongate mitochondria concentrated in basal processes and oriented vertically to basal surface Basal striations and apical brush border characteristic of proximal convoluted tubule At the very base of proximal convoluted tubule cell, interdigitating processes are actin filaments that may play a role in regulating movement of fluid from basolateral extracellular space across tubule basal lamina toward adjacent peritubular capillary 180 L/day of ultrafiltrate enters nephrons, and about 120 L/day is reabsorbed by proximal convoluted tubule 2 major proteins responsible for fluid reabsorption in proximal convoluted tubules o Na+/K+-ATPase pumps – localized in lateral folds of PM – responsible for reabsorption of Na+, which is major driving force for reabsorption of water in proximal convoluted tubule Active transport of Na+ into lateral intercellular space followed by passive diffusion of Cl-, and accumulation of NaCl in lateral intercellular spaces creates osmotic gradient that draws water from lumen into intercellular compartment, which distends as amount of fluid increases Lateral folds separate to allow distension o AQP-1 – functions as molecular water channel in PM of proximal convoluted tubules Hydrostatic pressure builds up in distended intercellular compartment, aided by contractile activity of actin filaments in base of tubule cells o This pressure drives essentially isosmotic fluid across tubule basement membrane into renal connective tissue, where the fluid is reabsorbed into vessels of peritubular capillary network Microvilli of proximal convoluted tubule cells covered with well-developed glycocalyx that contains several ATPases, peptidases, and high concentrations of disaccharidases Ultrafiltrate also contains small peptides and disaccharides o Disaccharides adsorb on glycocalyx for further digestion before internalization of resulting amino acids and monosaccharides (including glucose) Amino acid and glucose resorption in proximal convoluted tubule depends on active Na+ transport Deep tubular invaginations present between microvilli of proximal convoluted tubule cells (these invaginations have the glycocalyx that proteins in ultrafiltrate bind to) o Endocytotic vesicles containing bound protein bud from invaginations and fuse in apical cytoplasm to form large protein-containing early endosomes, which will become lysosomes and endocytosed proteins will be degraded by acid hydrolases o Amino acids produced in lysosomal degradation recycled into circulation via intercellular compartment and interstitial connective tissue pH of ultrafiltrate modified in proximal convoluted tubule by reabsorption of bicarbonate and secretion into lumen of exogenous organic acids and bases derived from peritubular capillary circulation Structure and Function of AQP Water Channels AQP’s are family of small, hydrophobic, transmembrane proteins that mediate water transport in kidney and other organs (liver, gallbladder, etc) Consists of 6 transmembrane domains arranged to form a distinct pore Most are selective for the passage of water o Aquaglyceroporins (AQP-3, AQP-7, and AQP-9) allow for transport of glycerol as well AQP-1 – expressed in proximal convoluted tubules of kidney, hepatocytes, RBC’s, lymph nodes, endothelial cells lining lymph sinuses, and on vascular endothelium of high endothelial venules – also in lacteals of endothelial cells in intestine AQP-2 – present in terminal portion of distal convoluted tubules and epithelium of collecting tubules and ducts o Under regulation of ADH o Mutation of AQP-2 is linked to congenital nephrogenic diabetes insipidus AQP-3 and AQP-4 – detected in basolateral cell surface of light cells of kidney collecting ducts Proximal Straight Tubule Cells not specialized for absorption Shorter, with less well-developed brush border, and with fewer and less complex lateral and basolateral processes Mitochondria smaller than those of cells of convoluted segment and are randomly distributed in cytoplasm Fewer apical invaginations and endocytotic vesicles Fewer lysosomes Thin Segment of Loops of Henle Length varies on location of nephron in cortex 2 types of thin segment tubules, one with more squamous epithelium than other Type I epithelium – found in thin descending and ascending limbs of loop of Henle of short-looped nephrons o Consists of thin, simple epithelium o Almost no interdigitations with neighboring cells and few organelles Type II epithelium – found in thin descending limb of long-looped nephrons in cortical labyrinth o Consists of taller epithelium o Abundant organelles and have many small, blunt microvilli o Extent of lateral interdigitation with neighboring cells varies Type III epithelium – found in thin descending limb in inner medulla o Consists of thinner epithelium o Simpler in structure and fewer microvilli than type II o No lateral interdigitations Type IV epithelium – found at bend of long-looped nephrons and through entire thin ascending limb o Consists of low, flattened epithelium without microvilli o Possess few organelles Ultrafiltrate that enters thin descending limb is isosmotic, and ultrafiltrate leaving thin ascending limb is hypotonic to plasma o Caused by reabsorbing more salts than water Thin descending limb – highly permeable to water and much less permeable to solutes like NaCl and urea o Because interstitial fluid in medulla is hyperosmotic, water diffuses out of this nephron segment o Small amount of NaCl and urea enters nephron o Cells do not actively transport ions, so increased tubular fluid osmolality caused by passive movement of water into peritubular connective tissue Thin ascending limb – does not actively transport ions, but is highly permeable to NaCl o Cl- diffuses into interstitium following its concentration gradient through Cl- conducting channels Energy from ATP required to open channels, but movement is passive o Counter ions (lots of Na+ and a little K+) follow Cl- passively to maintain electrochemical neutrality o Hyperosmolarity of interstitium directly related to transport activity of cells in thin ascending limb of loop of Henle o Largely impermeable to water, so as salt concentration increases in interstitium, interstitium becomes hyperosmotic and fluid in lumen of nephron becomes hyposmotic o Epithelial cells in thick ascending limb produce uromodulin (Tamm-Horsfall protein) that influences NaCl reabsorption and urinary concentration ability Uromodulin modulates cell adhesion and signal transduction by interacting with various cytokines Inhibits aggregation of calcium oxalate crystals (preventing kidney stones) and provides defense against UTI In individuals with inflammatory kidney diseases, precipitated uromodulin detected in urine as urinary casts Distal Straight Tubule Part of ascending limb of loop of Henle Includes both medullary and cortical portions (cortical portions in medullary rays) Transports ions from tubular lumen to interstitium Apical cell membrane has synporters that allow Cl-, Na+, and K+ to enter cell from lumen o Na+ actively transported across basolateral plications by Na+/K+-ATPase pumps o Cl- and K+ diffuse out from intracellular space by Cl- and K+ channels o Some K+ leaks back into tubular fluid through K+ channels, causing tubular lumen to be positively charged with respect to interstitium o Positive gradient provides driving force for reabsorption of Ca2+ and Mg2+ o Ion movement not accompanied by water, resulting in water separating from solutes Large cuboidal cells o Extensive basolateral plications o Numerous mitochondria associated with basal folds o Fewer and less well-developed microvilli than proximal straight tubule cells Distal Convoluted Tubule Responsible for o Reabsorption of Na+ and secretion of K+ into ultrafiltrate to conserve Na+ o Reabsorption of bicarbonate ions with concomitant secretion of H+, leading to further acidification of urine o Secretion of ammonium in response to kidneys’ need to excrete acid and generate bicarbonate Aldosterone (secreted by adrenal gland and released under stimulation by angiotensin II) increases reabsorption of Na+ and secretion of K+, increasing blood volume and pressure in response to increased blood Na+ concentration Collecting Tubules and Collecting Ducts Composed of simple epithelium (as are cortical collecting ducts and medullary collecting ducts) o Collecting tubules and cortical collecting ducts have flattened cells, somewhat squamous to cuboidal in shape o Medullary collecting ducts have cuboidal cells, with transition to columnar cells as ducts increase in size 2 types of cells present in collecting tubules and ducts o Light cells (collecting duct cells or CD cells) – principal cells of system Have true basal infoldings rather than processes that interdigitate with those of adjacent cells Possess single primary cilium and relatively few short microvilli Contain small, spherical mitochondria Possess abundance of ADH-regulated AQP-2 channels responsible for water permeability of collecting ducts AQP-3 and AQP-4 present in basolateral membrane o Dark cells (intercalated cells or IC cells) – have many mitochondria and denser cytoplasm Microplicae (cytoplasmic folds) present on apical surface, as well as microvilli Do not show basal infoldings but have basally located interdigitations with neighboring cells Numerous vesicles in apical cytoplasm Intercalated cells involved in secretion of H+ (α-IC’s) or bicarbonate (β-IC’s) depending on whether kidneys need to excrete acid or alkali α-IC’s actively secrete H+ into the collecting duct lumen via ATP-dependent pumps and releases HCO3- via Cl-/HCO3- exchangers located in basolateral cell membrane β-IC’s secrete bicarbonate ions into lumen of collecting duct there are more α-IC’s than β-IC’s cells of collecting ducts gradually become taller as ducts pass from outer to inner medulla and become columnar in region of renal papilla number of dark cells gradually decreases until there are none in ducts as they approach papilla Interstitial Cells interstitial tissue – connective tissue of kidney parenchyma – surrounds nephrons, ducts, and blood and lymphatic vessels much more of this in the inner region of the medulla and papilla than in the cortex in the cortex, there are two types of interstitial cells o cells that resemble fibroblasts – found between the basement membrane of tubules and adjacent peritubular capillaries – synthesize and secrete collagen and GAG’s of ECM of interstitium o macrophages In medulla, principal interstitial cells resemble myofibroblasts – oriented to long axes of tubular structures – contain prominent bundles of actin filaments, abundant rER, well-developed Golgi complex, and lysosomes – prominent lipid droplets in cytoplasm increase and decrease in relation to diuretic state Most fibroblasts originate in interstitial tissue through epithelial-mesenchymal transition o Conversion of tubular epithelial cells into mesenchymal phenotype – initiated by an alteration in balance of local cytokine concentrations o During chronic injury or inflammation, fibroblasts increase their numbers and, by secreting excess ECM, destroy normal interstitial architecture of kidney o Renal fibrosis – more than 1/3 of all disease-related fibroblasts originate from tubular epithelial cells at site of injury o Proliferation of fibroblasts in response to local mitogens usually leads to irreversible renal failure characterized by tubulointerstitial nephritis Histophysiology of Kidney Ability to excrete hyperosmotic urine depends on countercurrent multiplier system that involves o Loop of Henle – acts as countercurrent multiplier Osmotic gradients of medulla established along loop of Henle o Vasa recta – form loops parallel to loop of Henle and act as countercurrent exchangers of water and solutes between descending part (arteriolae rectae) and ascending part (venulae rectae) – helps maintain osmotic gradient of medulla o Collecting duct in medulla – acts as osmotic equilibrating device Modified ultrafiltrate in collecting ducts can befurther equilibrated with hyperosmotic medullary interstitium – level of equilibration depends on activation of ADH-dependent water channels (AQP-2) Countercurrent multiplier effect of loop of Henle – ions that may diffuse back into descending limb are transported back out again in ascending limb Efferent arterioles of renal corpuscles of most of cortex branch to form capillary network that surrounds tubular portions of nephrons in the cortex (peritubular capillary network) Efferent arterioles of juxtamedullary renal corpuscles form several unbranched arterioles that descend into medullary pyramid (arteriolae rectae) – make a hairpin turn deep in medullary pyramid and ascend as venulae rectae Descending arterioles and ascending venules called collectively vasa recta Arteriolae rectae form capillary plexuses lined by fenestrated endothelium that supply tubular structures at various levels of medullary pyramid Because loop of Henle is impermeable to water, but the time it reaches the distal convoluted tubules, the urine is hyposmotic o With ADH, distal convoluted tubules, collecting tubules, and collecting ducts are highly permeable to water, allowing the ultrafiltrate to become isosmotic (in the cortex) o In medulla, increasing amounts of water leave ultrafiltrate as collecting ducts pass through increasingly hyperosmotic interstitium on their course to papillae Vasa rectae running parallel to loop of Henle ensures that vessels provide circulation to medulla without disturbing osmotic gradient established by transport of Cl- in epithelium of ascending limb of loop of Henle o Form countercurrent exchange system because both arterial and venous sides of loop are thin-walled vessels that form plexuses of fenestrated capillaries at all levels in medulla o As arterial vessels descend through medulla, blood loses water to and gains salt from interstitium so that at tip of loop, deep in medulla, blood is essentially in equilibrium with hyperosmotic interstitial fluid o As venous vessels ascend toward corticomedullary junction, the process is reversed Hormonal Regulation of Collecting Duct Function ADH (vasopressin) – produced in hypothalamus and released from posterior lobe of pituitary gland – increases permeability of collecting duct to water, producing more-concentrated urine ADH acts on AQP-2 channels in epithelium of terminal portion of distal convoluted tubule, collecting tubules, and collecting ducts, but more significant action in collecting tubules and collecting ducts (see subpoints) o Translocation of AQP-2-containing intracytoplasmic vesicles into apical cell surface – short term effect – results in increased number of available AQP-2 channels at cell surface, thus increasing water permeability of epithelium o Synthesis of AQP-2 and their insertion into apical cell membrane – long term effect Increase in plasma osmolality or decrease in blood volume stimulates release of ADH (so does nicotine) Central diabetes insipidus (CDI) – copious, dilute urine in absence of ADH o Called nephrogenic diabetes insipidus if it is caused by a mutation in 2 genes encoding AQP-2 and ADH – kidney does not respond to ADH because of defective AQP-2 and ADH receptor proteins synthesized by collecting tubule and duct epithelial cells Excess water consumption can inhibit ADH release Sweating, vomiting, and diarrhea stimulate release of ADH to conserve water lost in such dehydrating activities Blood Supply Each kidney has a renal artery from the aorta, which branches in renal sinus and sends interlobar arteries into substance of kidney Interlobar arteries travel between pyramids as far as cortex and then turn to follow arched course along base of pyramid between medulla and cortex (arcuate arteries) Interlobular arteries branch from arcuate arteries and ascend through cortex toward capsule – interlobular arteries are midway between adjacent medullary rays, traveling in cortical labyrinth Afferent arterioles – branches off interlobular arteries as they traverse cortex toward capsule o These may branch off each other as well Some interlobular arteries terminate near periphery of cortex, whereas others enter kidney capsule to provide arterial supply Afferent arterioles give rise to capillaries that form glomerulus – these reunite to form an efferent arteriole that gives rise to the peritubular capillaries Efferent arterioles from cortical glomeruli lead into peritubular capillary network that surrounds local uriniferous tubules Efferent arterioles from juxtamedullary glomeruli descend into medulla alongside loop of Henle – break up into smaller vessels that continue toward apex of pyramid, but make hairpin turns at various levels to return as straight vessels toward base of pyramid – give rise to vasa recta and their peritubular capillary network Veins run parallel to arteries but flow in reverse direction Peritubular cortical capillaries drain into interlobular veins, which drain into arcuate veins, interlobar veins, and renal vein Medullary vascular network drains into arcuate veins, etc… Peritubular capillaries near kidney surface and capillaries of capsule drain into stellate veins, which drain into interlobular veins, etc… Lymphatic Vessels 2 major networks of lymphatic vessels o One is located in outer regions of cortex and drains into larger lymphatic vessels in capsule o Other network located more deeply in substance of kidney and drains into large lymphatic vessels in renal sinus o These two have many anastomoses Nerve Supply Renal plexus – derived from SNS – cause contraction of vascular smooth muscle and consequent vasoconstriction Constriction of afferent arterioles to glomeruli reduces filtration rate and decreases production of urine Constriction of efferent arterioles from glomeruli increases filtration rate and increases production of urine Loss of SNS innervation leads to increased urinary output Extrinsic nerve supply not necessary for normal renal function Ureter, Urinary Bladder, and Urethra On leaving collecting ducts at area cribrosa, urine flows to a minor calyx, then a major calyx, then the renal pelvis, leaving the kidney through the ureter to the urinary bladder o All of the above named organs have a mucosa (lined by transitional epithelium), muscularis, and adventitia (or serosa) Transitional epithelium (urothelium) lines excretory passages leading from kidney o Stratified epithelium – essentially impermeable to water and salts o Begins in minor calyces as 2 cell layers and increases to 4-5 layers in ureter – as many as 6 or more layers in empty bladder When bladder is distended, as few as 3 layers are seen Cells in distended bladder flatten and unfold to accommodate for increased SA Surface epithelial cells of empty bladder are usually cuboidal and bulge into lumen – “dome shaped” because of curvature at apical surface PM has plaques – more rigid and thicker than rest of apical PM – actin filaments stretch from the inner surface of these to a scalloped contour o Because cell folds inward on itself, plaques appear as series of fusiform vesicles o Lumina are in continuity with cell’s exterior o As bladder distends, fusiform vesicles unfold and become part of surface as cell stretches and flattens Dense collagenous lamina propria underlies urothelium o In tubular portions (ureters and urethra), 2 layers of smooth muscle lie beneath lamina propria Longitudinal layer – inner layer arranged in loose spiral pattern Circular layer – outer layer in tight spiral pattern o Smooth muscle of urinary passages mixed with connective tissue so that it forms parallel bundles rather than pure muscular sheets o Peristaltic contractions of smooth muscle move urine from minor calyces through ureter to bladder Luminal surface of wall of ureter – urothelium – rest of wall is smooth muscle and connective tissue o Smooth muscle arranged in 3 layers Inner longitudinal layer Middle circular layer Outer longitudinal layer o Outer longitudinal layer only present at distal end of ureter o Ureter embedded in retroperitoneal adipose tissue, which along with vessels and nerves, form adventitia of ureter As bladder distends, openings of ureter are compressed, reducing possibility of reflux of urine into ureters – contraction of smooth muscle of bladder wall also compresses openings of ureters to help prevent spread of infection from bladder and urethra (frequent sites of chronic infectin) to kidney In terminal portion of ureters, thick outer layer of longitudinal muscle present as well as other 2 Ureteric orifices – openings for ureters in bladder Internal urethral orifice – opening of urethra in bladder Trigone derived from embryonic mesonephric ducts, and major portion of wall originates from cloaca Detrusor muscle – smooth muscle of bladder wall o Contraction compresses entire organ and forces urine into urethra Internal urethral sphincter – ring-like arrangement of muscle around opening of urethra (muscle and collagen fibers randomly mixed) SNS fibers form plexus in adventitia of bladder wall and innervate blood vessels in the wall PNS fibers originate from S2-S4 and travel with pelvic splanchnic nerves into bladder, where they end in terminal ganglia in the muscle bundles and adventitia and are efferent fibers of micturition reflex Sensory fibers from bladder to sacral portion of spinal cord are afferent fibers of micturition reflex Male urethra has 3 parts o Prostatic urethra – from neck of bladder through prostate gland – lined with urothelium – ejaculatory ducts and prostatic ducts empty into posterior wall here o Membranous urethra – from apex of prostate gland to bulb of penis, passing through deep perineal pouch – skeletal muscle of deep perineal pouch forms external (voluntary) sphincter of urethra – urothelium ends here and this is lined with stratified or pseudostratified columnar epithelium that resembles epithelium of genital ducts o Penile (spongy) urethra – extends through penis and opens on body surface at glans penis – surrounded by corpus spongiosum and lined with pseudostratified columnar epithelium except at distal end, where it is stratified squamous (continuous with skin of penis) Ducts of bulbourethral glands (Cowper’s glands) and mucus-secreting urethral glands (glands of Littré) empty into penile urethra Female urethra runs from bladder to vestibule of vagina, where it terminates just posterior to the clitoris o Mucosa has longitudinal folds o Lining initially transitional epithelium (continuation of bladder epithelium) but changes to stratified squamous epithelium before its termination (may have stratified or pseudostratified columnar in the midportion) o Numerous urethral glands open into urethral lumen o Paraurethral glands (homologous to prostate gland) secrete into common paraurethral ducts, which open on each side of external urethral orifice and produce alkaline secretion o Lamina propria highly vascularized connective tissue that resembles corpus spongiosum o Where urethra penetrates urogenital diaphragm, striated muscle forms external urethral sphincter