Effects of Hematopoietic Cell Transplant

Phoenix Children’s Hospital Research
Institute (PCRI) Website Clinical Trial Listing
** Please complete and return to Shy Walker at swalker@phoenixchildrens.com
Study Title: Natural History and Biology of Long-Term Late Effects Following Hematopoietic Cell Transplant for Childhood Hematologic Malignancies
Sponsor: Center for International Bone Marrow Transplant Research (CIBMTR)
PCH IRB#: 14-104
Study Purpose (2-3 sentences in laymen terms):
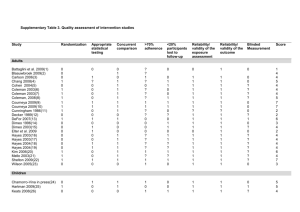
The primary objective of this study is to report the incidence of chronic kidney disease (CKD), metabolic syndrome, and osteopenia at one and two-years following allogeneic hematopoietic stem cell transplantation (HCT) for hematologic malignancy.
Study Summary (1 paragraph in laymen terms):
This is a prospective non-therapeutic study, assessing the long-term toxicity of pediatric HCT for hematologic malignancies. This study is a collaboration between the Pediatric Blood and Marrow Transplant
Consortium (PBMTC), the Center for International Blood and Marrow Transplant Research (CIBMTR), the
National Marrow Transplant Program (NMDP) and the Resource for Clinical Investigation in Blood and
Marrow Transplantation (RCI-BMT) of the CIBMTR. The study will enroll pediatric patients who undergo myeloablative HCT for hematologic malignancies at PBMTC sites.
Basic Eligibility Criteria (1 paragraph in laymen terms):
1. Age less than 22 years at admission for HCT. There is no lower limit on age.
2. Planned allogeneic HCT from any donor and stem cell source.
3. Patient must be in remission of one of the following diseases: acute lymphoblastic leukemia/lymphoma, myelodysplasia, acute myelogenous leukemia, juvenile myelomonocytic leukemia, chronic myelogenous leukemia.
Study Location(s): Phoenix Children's Hospital, main campus
Study Contact(s): Principal Investigator: Kristen Beebe, PA-C kbeebe@phoenixchildrens.com
Study Coordinator: Alan Holzkopf, CCRC aholzkopf@phoenixchildrens.com