Samples
advertisement

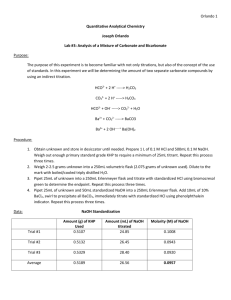
3 General Types of reactions in solution 1. Precipitates. These reactions are ideal for introducing the concept of the net ionic reaction. We use the rules of solubility to help us determine what happens but what often happens is that an insoluble compound is formed from other compounds that may be soluble. For example: Lead is pretty insoluble but all nitrates are soluble so I may have a solution in which lead (II) nitrate is mixed with potassium bromide solution. Lets assume 125 ml of 0.20 M lead (II) nitrate is mixed with 78.0 ml of 0.50 M potassium bromide for the reacting amounts. What will happen? The “normal” reaction The “complete ionic” reaction The “net ionic” reaction. Determine the amounts reacting Find the limiting reactant ( if any) Find the number of moles formed What is the best way to express the amounts? AP Chemistry - hendricks Solutions 1 2/8/2016 3 General Types of reactions in solution 2. Acid-Base. Electrolytes are those compounds that form ions in solution. They are called electrolytes because when ions float around in solution, the solution will begin to conduct electricity. There are 3 types of electrolytes: 1) acid (the pos ion is H+1) 2) base (the neg ion is -1OH) 3) salts (the pos ion is NOT H+1 and the neg ion is NOT -1OH) Again, we can use ionic form or net ionic form for the reaction and it is often much easier for us to do so. All acids you will deal with for now will have H+1 as the positive ion in solution. And the bases will produce -1 OH ion. These are the 2 half s of the water molecule so they will form water in solution and some other compound classified as a salt. Lets assume 25.2 ml of 1.25 M nitric acid is mixed with 24.2 g potassium hydroxide solid for the reacting amounts. What will happen? The “normal” reaction The “complete ionic” reaction The “net ionic” reaction. Determine the amounts reacting Find the limiting reactant ( if any) Find the number of moles formed What is the best way to express the amounts? AP Chemistry - hendricks Solutions 2 2/8/2016 3 General Types of reactions in solution 3. Redox. Redox reactions are the same as other reactions when using the coefficients to predict outcome from a reaction. The hard part for these is the technique used to balance them to begin with is often quite involved. You should be prepared to redox balance in either acid or base solution and to identify: a. All oxidation numbers for elements in either compounds or ions. b. the reduction half reaction c. The oxidation half reaction d. The agents for each e. The min number of electrons needed to make the reaction work f. How to use half reaction coefficients in the overall reaction g. How to balance the reaction in acid h. How to balance the reaction in base. Lets assume 12.3 ml of 0.663 M potassium permanganate is mixed with 43.0 ml of 0.252 M sodium sulfite. Later in the year you will be able to determine that the products will be Mn+2 ion and SO4-2 ion . If water was removed you may get some potassium sulfate and some sodium sulfate but lets assume water isn’t removed: The “net ionic” reaction. The reduction half reaction: The oxidation half reaction: The balanced net ionic reaction from the half reactions Oxidizing agent: ________________ reducing agent:______________ Balance in acid solution: Oxygens with water and Hydrogens with H+1 AP Chemistry - hendricks Solutions 3 2/8/2016 3 General Types of reactions in solution Balance in base solution: Add the same number of -1 OH to both sides and look for the net effect Determine the amounts reacting Find the limiting reactant ( if any) Find the number of moles formed What is the best way to express the amounts? AP Chemistry - hendricks Solutions 4 2/8/2016 3 General Types of reactions in solution Solutions Lab 1 – standardization of NaOH with KHP. The concentration of a solution of NaOH by using a specific mass of KHP, a monoprotic acid. 1 KHP + 1 NaOH 1 NaKP + 1 H2O Moles KHP mol NaOH grams KHP ml NaOH n/a M NaOH -You will need to make up a solution which is ~ 0.100 M. It doesn’t have to be to exactly 0.10 M but it should be a concentration known to 3 sig digits. Solutions Lab 2 – Now that you know the concentration of the NaOH solution, you can use that solution in an acid – base titration to find the concentrations of 2 other acids. 1 H2SO4 + 2 NaOH 1 Na2SO4 + 2 H2O mol H2SO4 mol NaOH ml H2SO4 ml NaOH M H2SO4 M NaOH Solutions Lab 3 – Now that you know the concentration of the NaOH solution, you can use that solution in an acid – base titration to find the concentrations of 2 other acids. 1 HCl + 1 NaOH 1 NaCl + 1 H2O AP Chemistry - hendricks mol HCl mol NaOH ml HCl ml NaOH M HCl M NaOH Solutions 5 2/8/2016 3 General Types of reactions in solution Wednesday Lab - Precipitation lab Gravimetric Analysis of a Metal Carbonate (Almost the same problem as your HW problem) Background: In this experiment, an unknown alkali metal carbonate, M2CO3, is analyzed to determine the identity of the metal. A known amount of the soluble unknown carbonate is dissolved in water to dissociate the compound into ions. M2CO3(s) 2M+(aq) + CO32-(aq) When a solution of calcium chloride, CaCl2, is added to this metal carbonate solution, a precipitate of calcium carbonate forms. Ca2+(aq) + CO32-(aq) CaCO3(s) The overall reaction represents a double-displacement reaction with a precipitate formed CaCl2(aq) + M2CO3(aq) CaCO3(s) + 2MCl(aq) The precipitated calcium carbonate is then filtered, dried, and weighed. The molar mass of the alkali metal carbonate can be determined using stoichiometry. Purpose: To determine the identity of an alkali metal carbonate using gravimetric analysis of a double-displacement precipitation reaction. Materials: Unknown metal carbonate Electronic balance Calcium chloride solution, 0.2 M Beakers, 400-mL Distilled water Drying oven Ring stand Filter paper Filter funnel Clay triangle Stirring rods Tongs Bunsen burner Watch glass Crucible Procedure: Dehydrating the Alkali Metal Carbonate 1. Prepare the crucible by heating for approximately 1 minute, allowing it to cool, and finding its mass. 2. Add approximately 2 g of the unknown carbonate to the crucible and find the combined mass of the crucible and carbonate. 3. Heat the crucible and carbonate form 2-3 minutes. Cool and mass. 4. Repeat step 3. Conducting the Precipitation Reaction 5. Add the crucible contents to a 400-mL beaker. 6. Add about 125 mL of 0.2 M CaCl2 and stir. Let the precipitate settle (5 min). 7. Filter out the precipitate using a piece of massed filter paper. 8. Place the filter paper on a watch glass and place in a drying oven for 10-15 minutes. 9. Remove and find the mass of the sample. 10. Break up the solid and return to the drying oven for 5 minutes. 11. Find the mass of the sample. Data/Observations: Value Mass (g) AP Chemistry - hendricks Solutions 6 2/8/2016 3 General Types of reactions in solution Mass of crucible Mass of crucible + M2CO3 Mass of crucible + M2CO3 (dried) Mass of crucible + M2CO3 (dried – 2nd time) Mass of filter paper Mass of filter paper + CaCO3 (1st time) Mass of filter paper + CaCO3 (2nd time) Calculations and conclusions: AP Chemistry - hendricks Solutions 7 2/8/2016 3 General Types of reactions in solution Friday lab – Redox reactions: some of these require the fume hood NO3 -1 ( aq) + Cu (s) Cu +2 (aq) + NO (g) ( you did this already ) Cr2O7 -2 (aq) + Fe +2 (aq) MnO4 -1 (aq) + Cl-1 (aq) Cl2(g) + MnO2(s) MnO4 -1 (aq) + Cl-1 (aq) Cl2(g) + Mn +2 AP Chemistry - hendricks Cr +2 (aq) + Fe +3 (aq) (aq) Solutions 8 2/8/2016 3 General Types of reactions in solution Yet another set of test samples for Monday with emphasis on titrations 4. Blood test for alcohol can be done with solution of dichromate (Cr2O7-2) ion that oxidizes the ethanol to carbon dioxide and water and reduced the Chromium to Chrome III (Cr +3 ) The unbalances reaction is shown below: C2H5OH (aq) + Cr2O7-2 (aq) CO2 (g) + H2O (l) a. Redox balance this reaction in acid and in base. In which solution is this reaction likely to be conducted? b. If 13.0 ml of 0.050 M dichromate solution is used to titrate a sample of blood plasma for alcohol, how much alcohol (grams) is in the plasma? c. If this alcohol came from a 10 ml sample of plasma with a density of 1.2 g/ml. Is this person legally drunk? 5. Some plants are poisonous because they produce Oxalic acid (H2C2O4 – diprotic) . Oxalate ions cause swelling of the respiratory tract and resulting suffocation. Assume 2.0 ml of plant extract from Rhubarb roots (an oxalic acid producer) is titrated against 22.1 ml of 0.1 M KOH solution. a. Show the reaction that occurs. b. What is the concentration of oxalic acid in that extract? 6. A common way to reduce the effects of Oxalate ion is to make it insoluble by drinking milk. a. What is in the milk that could make toxic compounds insoluble? b. Show the net ionic reaction that forms this precipitate from solvated oxalate ion. c. 200 ml of Milk provides about 400 mg of Calcium. How much milk would be required to neutralize the effect of the 2.0 ml of Rhubarb extract? AP Chemistry - hendricks Solutions 9 2/8/2016