Chemistry Summative Exam Part 2 Study Guide Answer Key
advertisement

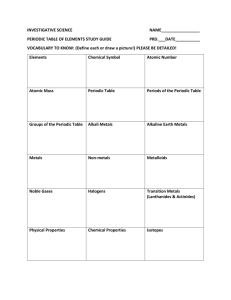
Chemistry Part 2 Summative Test study guide B – ANSWER KEY Define the following terms: 1. 2. 3. 4. 5. 6. Atom - the smallest particle of an element Electron – The negatively charged subatomic particle of an atom Nucleus - The center region of the atom where protons and neutrons are located Proton - The positively charged subatomic particle of an atom Neutron - The electrically neutral particle of an atom Atomic Number - The unique number that identifies the element and also the amount of protons and electrons found in the atom. 7. Atomic Mass – the average mass of the protons and neutrons in an atom. 8. Element symbol – The letter that represents the element on the periodic table of elements Look at the Fluorine on your periodic table and identify the number of 9. 10. 11. 12. 13. 14. 15. 16. 17. Protons 9 – (Equals the atomic number) Electrons 9 – (Equals the atomic number) Neutrons 10 – (Equals the atomic mass minus the atomic number) Draw a model of fluorine atom making sure to label the charge of each subatomic particle. Also be sure to label the nucleus. What are the physical properties of metals? Malleable, Ductile, High Density, Conductive What are the chemical properties of metals? Corrosive, Reactive What are the physical properties of non –metals? Low density, poor conductors, dull, brittle What are the chemical properties of non – metals? Some are reactive, some are not What elements are the most reactive and where are they located on the periodic table? The most reactive elements are the alkali metals located in the first family of the periodic table of elements. The column all the way to the left of the periodic table. 18. What elements are the least reactive and where are they located on the periodic table? The least reactive elements are the noble gases which are located in the last family of the periodic table of elements. The column all the way to the right of the periodic table of elements. 19. Where are the noble gases located on the periodic table? The column all the way to the right of the periodic table of elements 20. Where are the metals located on the periodic table? The metals are located all the way to the left and over to the metalloids portion of the periodic table. Chemistry Part 2 Summative Test study guide B – ANSWER KEY 21. Where are the non-metals located on the periodic table? 22. Where are the metalloids located on the periodic table? Define the following terms: 23. Element - the pure substance that cannot be broken down into any other substances by chemical or physical means 24. Compound - is a pure substance made of two or more elements that are chemically combined in a set ratio. 25. Chemical formula – show the elements in a compound and the ratio of atoms. Chemistry Part 2 Summative Test study guide B – ANSWER KEY 26. Chemical equation – is a short, easy way to show a chemical reaction, using symbols instead of words. 27. Chemical reaction – A change in matter that produces one or more new substances 28. Reactants - The substances you have in the beginning of your reaction. 29. Products - The substances you have at the end of your reaction 30. Law of conservation of matter – The principle of the law of conservation of mass / matter that in a chemical reaction, the total mass of the reactants must equal the total mass of the products Draw a model of the following molecule: H2O + Na(HCO3) CO2 + NaOH + H2O 31. Identify the products in the chemical reaction above: NaOH (Sodium Hydroxide) + H2O (Water) 32. Identify the reactants in the chemical reaction above. H2O (Water)+Na(HCO3) (Sodium Bicarbonate) 33. Prove that the above equation proves the law of conservation of matter. 34. Describe the factors that can affect the rate of a chemical reaction. Surface area, Temperature, concentration, catalysts and inhibitors