Measurement of Gaseous Diffusivities
advertisement
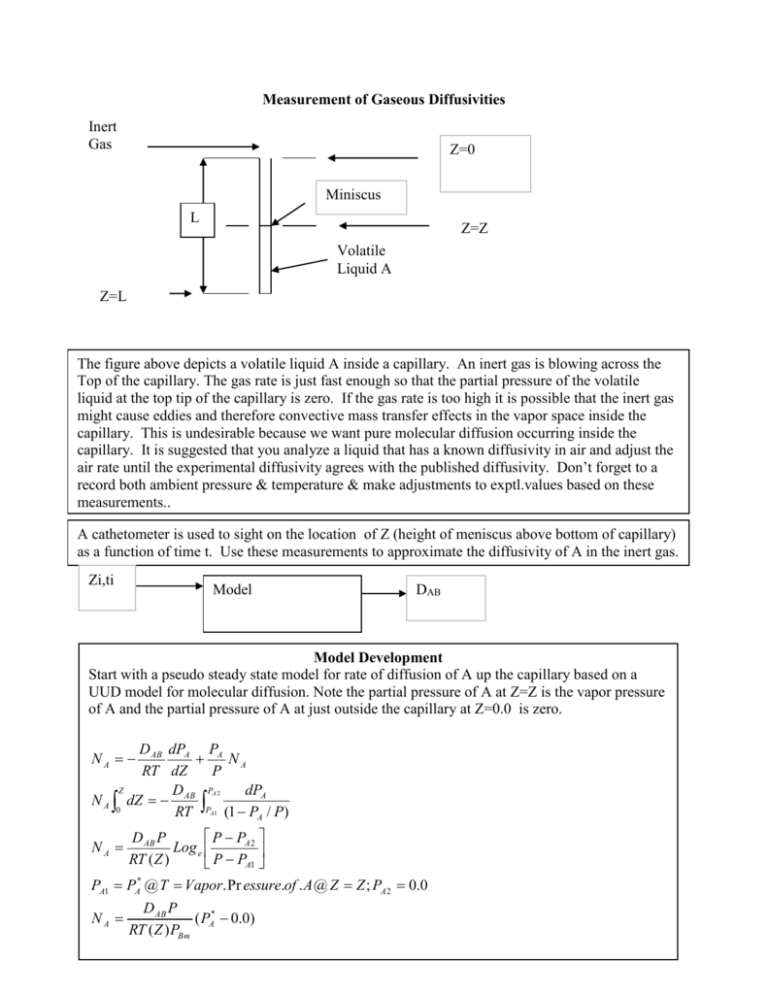
Measurement of Gaseous Diffusivities Inert Gas Z=0 Miniscus L Z=Z Volatile Liquid A Z=L The figure above depicts a volatile liquid A inside a capillary. An inert gas is blowing across the Top of the capillary. The gas rate is just fast enough so that the partial pressure of the volatile liquid at the top tip of the capillary is zero. If the gas rate is too high it is possible that the inert gas might cause eddies and therefore convective mass transfer effects in the vapor space inside the capillary. This is undesirable because we want pure molecular diffusion occurring inside the capillary. It is suggested that you analyze a liquid that has a known diffusivity in air and adjust the air rate until the experimental diffusivity agrees with the published diffusivity. Don’t forget to a record both ambient pressure & temperature & make adjustments to exptl.values based on these measurements.. A cathetometer is used to sight on the location of Z (height of meniscus above bottom of capillary) as a function of time t. Use these measurements to approximate the diffusivity of A in the inert gas. Zi,ti Model DAB Model Development Start with a pseudo steady state model for rate of diffusion of A up the capillary based on a UUD model for molecular diffusion. Note the partial pressure of A at Z=Z is the vapor pressure of A and the partial pressure of A at just outside the capillary at Z=0.0 is zero. D AB dPA PA NA RT dZ P Z PA 2 D dPA N A dZ AB 0 RT PA1 (1 PA / P) NA NA P PA 2 D AB P Log e RT ( Z ) P PA1 PA1 PA* @ T Vapor. Pr essure.of . A @ Z Z ; PA 2 0.0 NA D AB P ( PA* 0.0) RT ( Z ) PBm 1 Now address the unsteady State portion of the model Observe that the diffusion flux causes the liquid level to decrease in the capillary that has a cross sectional area Ar. Therefore, N AAr Z Zo (Z ) dZ A MA Ar dZ D AB PAr ( PA* @ T 0.0) dt RTPBm ( Z ) D AB PPA* M A (t 0.0) RTPBm A 2 D AB PPA* M A (Z Z ) t RTPBm A Now measure Z as function of time & plot (Z2-Zo2) vs time, i.e., 2 2 o Z2-Zo2 Slope= 2 D AB PPA* M A RTPBm A t=Time Report Format 1) Title Page + Table of Contents(10%) 2) Research binary gaseous diffusivity. Summarize this research with proper referencing. (10%) 3) Diffusivity measurement: Research & summarize methods for measuring the diffusivity of gases in binary systems. Use proper referencing. (15%) 4) Experimental Plan: Make an appropriate drawing and describe the procedure that you used to determine the diffusivity of a pure gaseous component in air. Your experimental plan must include studying the following: different pure component using the Arnold Cell. Don’t forget the effect of temperature? What was the ambient pressure and temperature during this experiment? Is it 2 important to know how this pressure & temperature varied during the experiment? Explain how you assured that temperature was held constant or you accounted for the variation in temperature during an experiment in your data analysis. (15%) 5) Tabulate your experimental data (an appropriate linear measurement & temperature & ambient pressure vs time) in a spreadsheet & apply appropriate regression analysis . Based on this statistical analysis what is the +/-% uncertainty in your reported diffusivity of a pure component in air. (35%) 6) Recommendation: Describe how you could improve your experimental procedure. Also describe another type experiment you might perform (including required equipment) that involves gaseous diffusivity of binary systems. (15%) Notes on Measurement of Gaseous Diffusivities with an Arnold Cell Ulus has set up the Arnold Cell with a capillary mounted inside a test tube with a side arm in the 17-1123 lab. Regulated air flows into the side arm of the test tube. A camera has been mounted in front of the Arnold cell to facilitate viewing the location of the vapor liquid interface inside the capillary. You must adjust the air rate so that it is not excessive. If you have too much air flowing, mass transfer will be occurring by a combination of molecular diffusion and convection. This is not desired for an experiment that is attempting to measure a gaseous molecular diffusion coefficient. Please also note that you need to have sufficient air flowing so that concentration at the top of the capillary of the species diffusing up the capillary is zero. Here is a potential list of chemical you may select from for this experiment. Remember you should measure the diffusivity of a pure component in air. You are required to look up & read carefully the MSDS sheet for the chemicals you are using. Select the chemicals that are least hazardous. It may be necessary to locate the apparatus in a hood if the chemicals are volatile & the MSDS indicates potential problems with exposure. Don’t forget to make multiple temperature measurements and ambient pressure measurements during an experiment. n-propanol Iso-propanol Ethyl Acetate Ethanol Acetone methanol 3 Title Page California State Polytechnic University, Pomona Chemical and Materials Engineering CHE 313- MASS TRANSPORT SPRING QUARTER, 2012 EXPERIMENT #1 Measurement of Gaseous Diffusivities INSTRUCTOR: T. K. Nguyen SECTION 01 GROUP #1 MEMBERS: Lau, Lee McCaffrey, Charles DUE DATE: May 10 2013 Title Page (10) ______ Research binary gaseous diffusivity (10) ______ Diffusivity measurement (15) ______ Experimental Plan (15) ______ Tabulated Data & Analysis (35) ______ Recommendation (15) ______ TOTAL (100) ______ 4