MLB - Core Courses -3.21- Kinetic processes in materials
advertisement

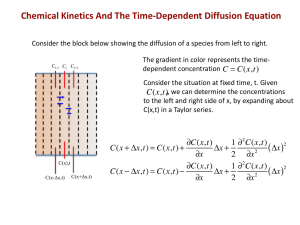
MLB - Core Courses -3.21Kinetic processes in materials Jean-Philippe Péraud Course 2 May 4th, 2012 1 Mass Diffusion in general -Mathematical concepts -Diffusion equation and associated phenomena -Solution methods 3 main parts Diffusion processes -relate microscopic effects to macroscopic equations and parameters Phase Transformations -continuous -discontinuous -kinetics of transformation -stability problems 2 Forces and fluxes • Force-flux relations force=-grad(potential) • Onsager • Constraint on quantities merges potentials. network constraint, electrochemical, elastochemical potential… 3 Diffusivities and frames *1 • Self-diffusivity *D 1 1 • Intrinsic diffusivity • Interdiffusivity, Darken equation 4 Interdiffusion & Kirkendall JA JV C-Frame JB A B v Vacancy sinks Dislocation shrink Vacancy sources Dislocation climb 5 Solution of diffusion equation: Toolbox Point source Step function Fourier series + superposition principle +method of images 6 Typical problems C=0 J=0 7 Diffusivity and random walks • Sequence of random jumps • Average displacement =0 • Average squared displacement proportional to D 8 Diffusivity and random walks • Simple models for frequency of jumps • More or less complicated depending on diffusion mechanism • Correlation factor 9 Diffusion in ionic crystals D Position depends on PO2 1/T • Kröger-Vink notation, Schottky, Frenkel defects • Be able to write the equation of incorporation of impurities • Use equation of equilibrium (Keq)+balance of charges • Identify diffusion regimes 10 Other diffusion mechanisms. In brief. • In grain boundaries • In amorphous materials • Polymers (by reptation) 11 Capillary phenomena: surface smoothing • By surface diffusion • By vapor transport +++ --12 Capillary phenomena: anisotropic surface tension 13 Capillary phenomena: coarsening and grain growth • Diffusion limited • Source-limited • N-6 rule Fluxes of atoms joining or leaving the particle shrinks grows 14 Continuous transformations: spinodal decomposition • Due to concave free energy profile in miscibility gap • Be able to explain CahnHilliard equation • Kinetics: use perturbation to derive critical and thermodynamic wavelength+amplification factor Credit: Balluffi, Allen, Carter, Kinetics of Materials 15 Continuous transformations: orderdisorder transformation • No energy barrier in concave up regions • Be able to explain AllenCahn equation • Kinetics: use perturbation to derive critical and thermodynamic wavelength+amplification factor Credit: Balluffi, Allen, Carter, Kinetics of Materials 16 Nucleation • Curve-to-curve and tangent to curve construction • Calculate Rc and ΔGc • Determine steady state rate. • Heterogeneous nucleation: almost the same thing 17 • Not covered: stability of moving interfaces 18 Advice • Get some sleep • Don’t panic • Always try to answer (partial credit) 19 Questions 20