Science Study Guide Chapter 12 Name: Which change below is a
advertisement

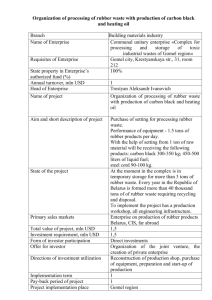
Science Study Guide Chapter 12 Name: ____________________________________ 1. Which change below is a chemical change? P374-375 One kind of matter changes phases. , One kind of matter keeps its identity and properties. , One kind of matter changes its shape and volume. , or One kind of matter changes into another kind of matter with different properties. 2. Which of the following is an example of combustion? P376-377 a log burning in a fire, a piece of gray metal turning to rust, a dry sponge expanding in water, or a container of milk souring to cottage cheese 3. Look at the chemical equation below. P378-379 C + 02 CO2 carbon + oxygen carbon dioxide Which is the product of the reaction? 4. Which of the following describes what happens during a chemical reaction? P378-379 Matter is destroyed. , Matter is created and destroyed. , Matter is not changed. , or Matter is neither created nor destroyed. 5. What kind of reaction occurs when two compounds split apart and the parts switch places, as shown in the reaction below? P 380-381 2 + H2S 6. Look at the illustration. P 380-381 What kind of chemical reaction is shown by the model? 7. Fossils can be found in limestone. Explain how scientists can use a chemical reaction to separate a fossil from limestone without damaging the fossil. P382-383 8. A scientist used universal indicator paper to test an unknown substance. The indicator paper turned red. What can the scientist conclude about the substance? p384-385 9. What bacteria-killing substance did Alexander Fleming accidentally discover that became an important life-saving medicine? P386-387 10. Look at the illustration. P388-389 This tent is form a polymer developed in a chemical laboratory. What is the name of the polymer? 11. Explain how treated rubber, which is heated and has sulfur added, has different properties from untreated rubber. Why is treated rubber more desirable? p390-391 12. Chemists use chemical technology to make new substances.p390-391 Part A Identify two materials that result from chemical technology. Part B Identify the properties the materials have that make them useful for human lives. 13. What should you do first before using chemicals at home? p 392-393