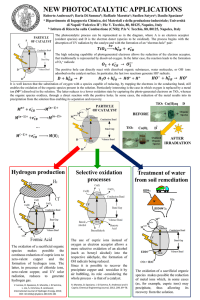
File
advertisement

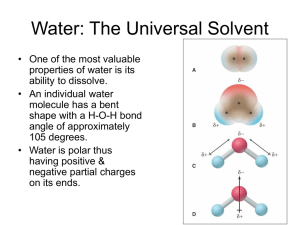
Chapter 4 – Types of Chemical Reactions and Solution Stoichiometry Section 4.1 – Water, the Common Solvent -the ability of water to dissolve many different substances depends on the chemical properties of the molecule -polar molecule: electrons and therefore charge are not distributed equally -hydration-process of water breaking apart a solute -ionic compounds break up into individual cations and anions -solubilities of ionic compounds in water usually depend on relative attractions of ions for each other versus the attractions of the ions for water molecules -water also dissolves nonionic substances (as long as they are polar molecules) -“like dissolves like” applies to polar and nonpolar dissolving abilities Section 4.2 – The Nature of Aqueous Solutions: Strong and Weak Electrolytes -substance=solute, liquid=solvent -aqueous solutions that conduct electricity very efficiently contain strong electrolytes -aqueous solutions that only conduct electricity weakly contain weak electrolytes -aqueous solutions that do not permit current to flow contain nonelectrolytes Strong Electrolytes -completely ionized when dissolved in water -three classes: soluble salts, strong acids, and strong bases -acids-substance that produces H+ ions (protons) when it is dissolved in water (Arrhenius) -strong acids-virtually every molecule ionizes -sulfuric acid is a special case-it has 2 protons to donate, but only the first one completely dissociates, so the solution contains H+ and HSO4-strong bases-ionic compounds containing the hydroxide ion Weak Electrolytes -ionize to a small degree, therefore produce few ions -most common weak electrolytes are weak acids and weak bases -weak bases produce hydroxide ions when dissolved in water, but do not actually contain the hydroxide ion (eg ammonia) Nonelectrolytes -dissolve in water but don’t produce any ions -usually molecular, rather than ionic, compounds, such as ethanol or sucrose Section 4.3 – The Composition of Solutions -in order to do stoichiometric calculations of reactions in solutions, we need to know what reaction is going on and the amount of chemicals in the solutions (concentrations) -molarity-moles of solute per volume of solution in liters -solution concentration is in terms of the solute before it was dissolved, ion concentration may be different based on the ionic compound -standard solution-a solution with an accurately known concentration Dilution -solutions often prepared as concentrated solutions (stock solutions) and then diluted later -remember that moles of solute after diluting = moles of solute before diluting -use volumetric and measuring pipets -M1V1=M2V2 Section 4.4 – Types of Chemical Reactions -almost all reactions can be placed into one of the three following categories: precipitation, acid-base, oxidation-reduction Section 4.5 – Precipitation Reactions -occurs when two solutions are mixed and a solid (precipitate) is formed -best way to identify the product is to figure out what products are possible -remember, when ionic solids are dissolved in water, they separate into their component ions -out of the products that are possible, find the one that is insoluble -knowing the colors of ions can be helpful -the soluble product remains as individual ions in solution -“slightly soluble” means that the amount of solid that dissolves is not noticeable, it is used with the term insoluble interchangeably at this level -solubility rules: -most nitrate salts are soluble -most salts containing the alkali metal ions and the ammonium ion are soluble -most chloride, bromide, and iodide salts are soluble, notable exceptions are solts containing Ag+, Pb2+, and Hg22+ -most sulfate salts are soluble, notable exceptions are BaSO4, PbSO4, HgSO4, and CaSO4 -most hydroxide salts are only slightly soluble, the important soluble hydroxides are NaOH and KOH -most sulfide, carbonate, chromate, and phosphate salts are only slightly soluble Section 4.6 – Describing Reactions in Solution -molecular equation is what we consider the “normal” chemical equation -doesn’t give a good picture of what is happening in the reaction -complete ionic equation breaks down all aqueous solutions into their component ions, leaves solids, liquids, and gases together -some ions remain the same on both sides of the equation-these are spectator ions -removing the spectator ions leaves the net ionic equation, which is what we want to use because it describes the reaction that is actually happening Section 4.7 – Stoichiometry of Precipitation Reactions -same principles apply in solutions as in regular stoichiometry problems -sometimes harder to figure out what reaction will occur when two solutions are mixed -to obtain moles of reactants, we need to use the volume used and the molarity of the solutions instead of just the mass -stoichiometry for reactions in solution: -identify the ions present in the solution and figure out what reaction occurs -write the balanced net ionic equation -calculate moles of reactants -determine limiting reactant -calculate moles of product(s) -convert to grams or other units Section 4.8 – Acid-Base Reactions -Arrhenius’ idea of acids and bases: acids produce H+ in water, bases produce OH-Brønsted-Lowry definition: acid is proton donor, base is a proton acceptor -to figure out what reaction will occur, use the same steps as precipitation reactions -if a solid will not form, check to see if water (a liquid, NOT aqueous) forms -calculations for acid-base reactions: -list the ions present before the reaction occurs, then decide what reaction will occur -write the balanced net ionic equation -calculate moles of reactants, using volumes and molarities if necessary -determine limiting reactant -calculate moles of reactant or product -convert to grams and volume -acid-base reactions also known as neutralization reactions Acid-Base Titrations -volumetric analysis: determining the amount of a substance using a titration -titrant: solution of known concentration -analyte: substance being analyzed -point at which enough titrant has been added to react exactly with the analyte is called the equivalence point or the stoichiometric point Section 4.9 – Oxidation-Reduction Reactions -reactions when one or more electrons are transferred Oxidation States -also known as oxidation numbers -helps keep track of electrons, especially in redox reactions -arbitrarily assigned electrons in covalent compounds based on compared electron affinity -assumes that electrons are usually shared unequally (which they are) -oxidation states assume a complete transfer of electrons as opposed to the sharing that actually occurs -rules for assigning oxidation states: -oxidation state of an atom in an element is 0 -oxidation state of any monatomic ion is its charge -oxygen has an oxidation state of -2 in covalent compounds except peroxides where each oxygen has an oxidation state of -1 -in covalent compounds with nonmetals, hydrogen has an oxidation state of +1 -in compounds, fluorine always has an oxidation state of -1 -the sum of oxidation states in neutral compounds must be zero, for an ion the sum must be the charge of the ion The Characteristics of Oxidation-Reduction Reactions -characterized by a transfer of electrons -transfer can be obvious (such as the formation of ions), other times, oxidation states must be determined for species on both sides of the reaction to see if and where electrons moved -oxidation: loss of electrons (increase in oxidation state), reduction: gain of electrons (decrease in oxidation state) -oxidizing agent is the electron acceptor, reducing agent is the electron donor -CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) -carbon is oxidized -oxygen is reduced -CH4 is the reducing agent (notice that the whole compound is named, not just the element) -O2 is the oxidizing agent Section 4.10 – Balancing Oxidation-Reduction Reactions Oxidation States Method of Balancing Oxidation-Reduction Reactions -assign oxidation states to each of the elements involved in the reaction -pay attention only to the species that change -add coefficients to balance out the number of electrons transferred during the reaction -after electrons are balanced, balance the rest of the equation the way that you would for any other chemical equation -when you are done balancing, you must double check that not only all elements balance on each side, but charge as well -you don’t need to show states of matter as you work through the balancing process, but your final balanced equation must include states of matter