Example
advertisement

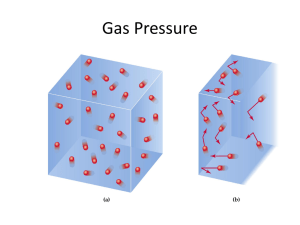
Chapter 10 - Gases Gas - a substance that is characterized by widely separated molecules in rapid motion. Mixtures of gases are uniform. Gases will expand to fill containers (compare with solids and liquids from Chapter 1). Common gases O2; N2 ; major components of "Air". O2; F2; Cl2; N2, H2 gaseous diatomic molecules; H2; He are both lighter than air; N2O (laughing gas) Note most molecular compounds are solids or liquids at room temperature, but they can be converted to a gas relatively easily. Ionic solids can't. Distinguish between a gas and vapour gases are normally in the gaseous state at 25°C and 1 atm pressure. Vapour is the gaseous form of any substance that is liquid or solid at normal temps. or pressure. The Definition of Pressure Pressure is best defined as the forces exerted by gas on the walls of the container Define P = force/area SI unit is the Pascal = N/m2 = (kg m s-2)/m2 = 1 kg / ms2 When chemists speak of the "pressure of a gas", they usually are speaking about the measurement of the gas relative to atmospheric pressure (Note change in gas pressure with distance from earth's surface) How do we measure gas pressure? Barometer - invented by Torricelli. unit of barometer measurement 1 Torr = 1mm Hg 2 1 atm pressure = 760mm Hg = 760 Torr NOTE; 1 atm is equivalent to 101,325 Pa = 1.01325 105 Pa = 1.01325 x 102 kPa Example of conversion between atm and mm Hg Antigonish N.S., atmospheric pressure is 100.7 kPa P (atm) = 100.7 kPa x 101.325 kPa/1 atm = 0.9938 atm We can use other liquids besides Hg (l) in a barometer for the measurements of atmospheric pressure. C2H5OH (one of my favourite examples) for 1 atm pressure, what would be the height of the liquid in the tube of CH3CH2OH is the liquid in the tube? (d (C2H5OH) = 0.798g/am3 Answer: The height difference would result from the difference in densities of the two liquids! height of CH3CH2OH = height of Hg x d (Hg) /d (CH3CH2OH) = 760mm Hg x 13.61 g/cm3 / 0.798gcm3 = 13.0 x 104 mm = 13.0 m The Gas Laws Regarded as useful summaries of the results of countless experiments. Boyle's Law Expresses the relationship between the volume and the pressure, .i.e., the volume occupied by the gas is inversely proportional to the pressure. Mathematically expressed as: V 1/P (constant T and number of moles Statement - The volume of a fixed amount of gas (at constant T, n) is 3 proportional to the inverse of the pressure or V = k1 x 1/P PV = k1 where k1 is a proportionality constant. What happens if we change the pressure of the gas? P1 = k1 x 1/ V k1 = P1 V1 = P2 V2 This relationship expresses directly the relationship between changing the pressure of gas (or volume occupied by gas) and the volume at the new pressure. Examples 1. A weather balloon has a volume of 400L at atmospheric pressure (1.0atm). The balloon is released to a height of 6.5 km where the press is 0.4 atm. What is the new volume of the balloon? P1 = 1.0atm V1 = 400 L P2 = 0.40atm V2 = ? Using Boyle's Law P1 V1 = P2 V2 V2 = V1 x P1/P2 V2 = 400L x 1.0atm/0.40atm = 1000 L = 1.0 x 103 L (sig. figs) 4 2. A gas, originality at V = 5.00L and P = 1.0 atm is expanded to a volume of 5.00. Calculate the new gas pressure! V1 V2 = 5.00L = 9.00L P1 = 1.0atm P2 = ? P1V1 = P2 V2 (5.00 x 1.0 atm) = P2 x 9.00L P2 = 5.00 L/9.00 L x 1.0 atm = 0.56atm 3. A sample of gas is placed in an evacuated bulb of V = 0.51 L and exerts a pressure of 24 kPa. This bulb is connected to another evacuated bulb whose volume is 0.63L. The gas is then allowed to fill both bulbs. What is the new gas pressure at room temperature? P1 = 24 kPa; P2 = ? V1 = 0.51L V2 = 0.51L + 0.63L = 1.14 L P2 = P1 V1/V2 = 24 kPa x 0.51L/1.14L = 10.7L = 11. L (sig. figs) Charles and Gay - Lussac's Law Defines the relationship between the gas volume and the temperature. At constant pressure, the volume of a fixed quantity gas is directly dependent on the absolute temperature of the gas (i.e. K degrees => Lord Kelvin's temp. scale). V = k2 x T (const. pressure) k2 a proportionality constant that depends on the pressure 5 An Aside The Kelvin Temperature Scale - Lord Kelvin recognized the significance of the intercept in the volume temperature relationship (all plots intercepted the t axis at -273.15°C). Kelvin termed this absolute 0, and it is the temperature where the volume of an ideal gas is 0 and all thermal motion ceases. For the Kelvin temperature scale, O K = -273.15°C T (k) = [ tC (°C) + 273.15°C] K/°C freezing point of water: t = 0 °C; T = 273.15 K boiling point of water: t = 100 °C; T = 373.15 K room temperature: t = 25 °C; T = 298 K NOTE t = C; T = K NO DEGREE SIGN. End of Aside 1. A gas allowed to expand (double) its volume against a constant opposing pressure of 1.0 atm. If the temperature of the gas initially was 298.15 K, what is the final temperature of the gas? V1 = V1 T1 = 298.15 K V2 = 2 V1 T2 = ? V1/ T1 = V2/T2; V1/ 298.15K = 2V1/T2 = 596. K 2. A sample of CO2 occupies 7.50 L at 150°C. If the pressure remains constant, calculate the temperature at which the CO2 will occupy 3.76 L. V1 = 7.60L V2 = 3.76L T1 = (273.15 + 150) K = 423.2 K T2 = ? V1/ T1 = V2/T2; V1/ 423.2 K = V2/ T2 T2 = V2 / V1 x 423.2 K = 3.76 L / 7.50 L x 423.2 K. = 212 K. What about the pressure - temperature relationship? 6 for a given quantity of gas at a fixed volume, we have P T, i.e., if we heat a gas cylinder, P increases! P = k3 T P1 = k3T1 P2 = k3T1 P1 / T1 = P2/T2 Amonton's Law Example If the gas pressure inside an aerosol can was 1.5 atm at 25°C, what would the pressure be if the can were heated to 425°C? P1 = 1.5 atm P2 = ? T1 = (25 + 273) K = 298K; T2 = (425 + 273) K = 698 K P2 = P1 x T2 / T1 = 1.5atm x 698K/298K = 3.5atm Amonton's Law #2 A car tire in Manitoba in the summer has a pressure of 220 kPa at a temperature of 25°C. In the winter, the tire pressure is measured at 176 kPa. Calculate the temperature of the air inside the tire. P1 = 220 kPa P2 = 176 kPa T1 = (25 + 273) K = 298K; T2 = ? P1 P2 = T1 T2 T2 = T1 x P2 P1 T2 298 K x176kPa 220kPa T2 238K 238K 273.15 35 o C Avogadro's Law The volume of a gas at constant T and P is directly proportional to the number of moles of gas. V = k4 n => n = number of moles of gas. e.g.,1 mole CO2 occupies 22.414 L at 273.15K, and 7 P = 1.000 atm; 2 moles of CO2 gas would occupy 44.86 L. The Ideal Gas Equation of State We have four relationships. V 1/P; Boyle’s Law V T; Charles’ and Gay Lussac's Law V n; Avogadro’s Law P T; Amonton’s Law can combine all these expressions into a single fundamental equation of state, called the ideal gas Law. Vn T V = P nRT or PV = nRT P where R is the gas constant = 0.082057 L atom / (K mol) An ideal gas is a gas that obeys totally the ideal gas law over its entire P-V-T range. Ideal gases don't exist, but a fair number of real gases approach ideal behavior over certain T, P, and V ranges. NOTE: Ideal gases - molecules have negligible intermolecular attractive forces and they occupy a negligible volume compared with the container volume. Define: STP (Standard Temp Pressure) O°C (273.15K) 1 atm (101.325KPa) 8 Calculate the volume occupied by 1.00 mole of an ideal gas at STP. T = 273.15 K ; n=1.00 mol P = 1.00 atm; P V = n RT 1.00 atm x V = 1.00 mol x 0.08206 L atm / (K mol) x 273.2 K V = 22.4 L Note that this is a general statement for any ideal gas; 1 mole of an ideal occupies 22.4136 L at STP. Other Examples of Calculations using the Ideal Gas Equation Recovering the Individual Gas Laws e.g., Boyle’s law PV = n R T or P1V1 = n R T (i.e., T ,n constant) P2V2 = n R T P1V1 = n R T = P2 V2 (Boyle’s Law). e.g., Charles’ and Gay Lussac’s Law PV = n R T or PV1 = n R T1 (i.e., P, n constant) PV2 = n R T2 V1T1 = n R / P = V2 T2 (Charles’ and Gay Lussac's Law). Calculate the volume occupied by 32.06 g of Ne (g) at 5.0°C and 630 mm Hg. P = 630 mm Hg x 1 atm/760 mm Hg P = 0.8289 atm = 0.829 atm T = (5 °C + 273 °C) 1 C / 1 K = 278. K n Ne = 32.06 g Ne x 1mol Ne / 20.18 g = 1.589 moles P V = n RT V = n RT / P = 1.589 moles x 0.08206 L atm / (K mol) x 278. K / 0.829 atm = 43.7 L 9 Gas Density Calculations Gas Law PV PV =nRT n = mass / molar mass = g / M = g/M RT or M = g R T/ (P V) but we know density = mass/volume = g/v M = (d R T) / P if we measure the density of a gas, we can calculate M a newly discovered gas has a density of 2.39g/L (STP). Calculate the molar mass of the new gas! STP T = 273.15K d = 2.39g / L P = 1.00atm M=dRT/P = 2.39g / L x 0.08206 L atm /K mole x 273.15 K / (1.00atm) = 53.6 g / mole Mixtures of Gases and The Concept of Partial Pressures So far, we have only talked about pure gases. In the real world, we are concerned mainly with mixtures of gases (two or more components). We need to be able to obtain a relationship between the total pressure of a mixture of gases and the pressure exerted by an individual gas in that mixture. How can we do this?? Let's consider two ideal gases (gas 1 and gas 2) in a container of volume V. From the ideal gas equation, the pressure exerted by gas #1 P1 = n1 R T / V Pressure exerted by gas #2 P2 = n2 RT/ V the total pressure of 1 and 2 on the container wall is then PT = P1 + P2 = n1 R T / V + n2 RT/ V = (n1 + n2) (RT/V) 10 = nT RT / V We define nT as the total number of moles of gas present in the mixture (i.e., nT = n1 + n2) and P1 and P2 are the partial pressures of gas 1 and gas 2, respectively. Since we can rewrite the above equation PT = P1 + P2 = nT (RT/ V), in general, we can write that PT = P1 + P2 + P3 +... where P1 is the partial pressure exerted in the container by gas 1, gas 2, etc. Dalton's Law of Partial Pressure In a gaseous mixture, each gas exerts the same pressure as if it was alone and occupied the same volume. Let's go back to our gaseous mixture: P1 / Pt = [n1 RT/V] / [(n1 + n2)RT/V] = n1 / (n1 + n2) = X1 where X1 is defined as the mole fraction of gas 1 Note that the mole fraction is a dimensionless quantity; it simply gives the ratio of the number of moles of gas 1 to the total number of moles of gas present in the mixture. partial pressure of gas 1 = P1 = X1 x PT partial pressure of gas 2 = P2 = X2 x PT In general, in gaseous mixtures, the partial pressure of the ith component is related to the total pressure by Pi = Xi PT where Xi is the mole fraction of gas i. Example Assume 1.00 mole of air molecules contains about 0.78 mole N2, 0.21 mole 02, and 0.010 mole Ar. Calculate the partial pressures of each gas in the mixture when the total air pressure 1.0 atm. Sol'n PT = Pair = nT RT/ V = (nN2 + n02 + nAr) RT/V 11 from the partial pressure Pi = Xi PT we need to calculate the mole fraction of gas in the mixture, Xi = ni/nT . for O2 XO2 = nO2 / nair = 0.21 mole /1.0mole = 0.21 for N2 XN2 = nN2 / nair = 0.78 mole /1.0mole = 0.78 we can calculate XAr in two ways (i) XAr = nAr / nair = 0.010 mol/1.0 = 0.010 or (ii) Xar + XN2 + XO2 = 1.00 Partial pressures PO2 = 0.21 x1.0 atm = 0.21atm PAr = 0.010 x 0.010 atm = 0.010 atm PN2 = 0.78 x 1.0 atm = 0.78atm Many gas measurements are carried out over water. We also collect water vapour with the gas. The water vapour pressure must be subtracted from the total pressure to give the pressure of gas collected. Example O2 gas is generated in a reaction and collected over water at 24°C and 762 mm Hg to in a vessel whose volume is 128 mL. Calculate the mass of O2 obtained given that that the vapour pressure of water is 22.4mm of Hg at 24°C. Solution we need to calculate the pressure of the gas that is due to the O2 ONLY. PT = PO2 + PH2O PT = 762 mm of Hg = PO2 + PH2O PO2 = 762 mm of Hg - 22.4 mm of Hg = 740 mm of Hg (remember sig. figs.) PO2 (atm) = 740 mm of Hg x 1 atm / 760 mm Hg = 0.974 atm Next from ideal gas law PV = n R T 12 T (K) = (273.15C + 24. C) x 1K / 1 C We can calculate the number of moles of O2 gas collected over water by n = P V / R T = 0.974 atm x 0.128L/0.08206 L atm/ K mol x 297.15K = 0.00512 moles of O2 n = g / molecular mass g O2 = n x 32.00 g / mol = 0.164 g of O2 (g) collected. Kinetic Molecular Theory of Gases So far we have talked about macroscopic (i.e., large quantity) behaviour of gases. Kinetic molecular theory of gases has been developed by many scientists (principally Maxwell, Boltzmann, and Clausius) over a number of years to explain the regular behaviour of all gases (e.g. gas laws). This theory explains the behaviour of gases on a molecular level. Gas Laws Explanations gas pressure results from the collisions of gas molecules with the container walls. The pressure is dependent on both the number of collisions and how hard gas molecules strike the wall! Kinetic Theory of Gases 1. Gases consist of molecules widely separated in space. Volume of molecules is negligible compared to total gas volume. 2. Gas molecules are in constant, rapid, straightline motion. Collisions are elastic. 13 3. Average kinetic energy (K.E.) of molecules depends on absolute temperature (T) only. 4. Attractive forces between molecules are negligible. 14 collision of gas molecule with container wall results in pressure 1. Let's increase the amount of gas in the container (T, V constant) more collisions of gas with container wall and the pressure increases. (c.f., from the ideal gas equation, V n at constant P, T). 2. Let's decrease the volume of the container (constant n and T). We still have the same number of gas molecules, but now the volume of the container is smaller (we push down the piston). more collisions of the gas molecules with the container wall and P increases. (Boyle's Law V 1/P) 3. Let container volume increase (P, n are held constant). According to Kinetic Molecular Theory, in order for the pressure to stay constant, the number of gas collisions per second must increase (otherwise P will decrease). Hence, the molecules must move faster, and T will increase. Kinetic Molecular theory says K.E. increases as T increases. (Explains Charles’ and Gay-Lussac’s law). Molecular Speeds K.E. = 1/2 M U2; note that this speed is an average speed (some will always be fast, some slow). Analogy with a fast freeway; some cars drive fast on the freeway, some will drive slower. relationship between molecular speed and mass. A useful result of K.M.T. of G is that we can relate macroscopic measurements to molecular processes (i.e., P, V to Molecular mass and mass square seed u2) e.g. P V = 1/3 n M u2 = n R T 1/3 n M = n R T => solving for u2 u2 = 3RT/M or u2 = 3 R T/ M this is the mean square speed. Example: What is the rms speed for Ne at 298 K. 15 -3 Ne M = 20.18 g = 20.18 x 10 kg/mol u rms 3RT MNe J x298 K K mol 20.18 x10 3 kg / mol 3 x8.314 urms = 607 m/s Compare this speed with that of He (g) urms 3RT MHe J x298 K K mol 4.003 x10 3 kg / mol 3 x8.314 for He, urms = 1363 m/s = 1.36 x 103 m/s We note that as gas molecules travel through space, they will encounter collisions with other gas molecules and with the walls of the container. Define - mean free path as the average distance between successive molecular collisions. we note that as the pressure of the gas increases, the mean free path decreases, i.e., the higher the pressure, the greater the number of collisions encountered by a gas molecule. Effusion and Diffusion Diffusion - gradual mixing of gas molecules caused by kinetic properties. Graham's Law => Under constant T, P, the diffusion rates for gaseous substances are inversely proportional to the squares roots of the molar masses. 16 r1 M2 r2 M1 where r1 and r2 are the rates of diffusion of gases 1 and 2 and M1 and M2 represent the molar masses of gas 1 and gas 2, respectively. Effusion - the process by which a gas under pressure goes (escapes) from one compartment of a container to another by passing through a small opening. t2 M2 t1 M1 where t1 and t2 are the effusion times of gases 1 and 2 and M1 and M2 again represent the molar masses of gas 1 and gas 2, respectively. How quickly the molecules diffuse depends on their molecular speed and the mean free path (i.e., the collision frequency). Examples of Graham's Law problems Calculate the diffusion ratio of hydrogen gas to oxygen gas! M (H2) = 2.016 x 10-3 kg/mole; M (O2) = 32.00 x 10-3 kg/mole r1 M2 r2 M1 32.00 x10 3 kg / mole 4 2.016 x10 3 kg / mole on average, H2 molecules move 4x faster than O2 molecules. Example #2 In an effusion apparatus, pure O2 required 55.0 min to reach the detector. Under the same conditions, an unknown gas needed 85.0 min. Calculate the molecular mass of the unknown gas. 17 t unk Munk 85.0 t O2 MO 2 55.0 Munk 32.00g / mole 2 2 Munk Munk 85.0 1545 2 . 55.0 32.00 g / mole 32.00 g / mole 2.388 = Munk Munk 76.4 g / mole 32.00 g / mole Deviations from Ideal Gas Behaviour The ideal gas equation of state is not sufficient to describe the P,V, and T behaviour of most real gases. By rearranging the ideal gas equation, the degree to which a real gas departs from ideal behaviour may be seen. Most real gases depart from ideal behaviour at deviation from at a) Low T b) High P look at assumptions for ideal gas 1. Real gas molecules do attract one another. At high pressures, the intermolecular distances decrease, and the gaseous molecules experience significant attractive forces. Hence, P is decreased from that of an ideal gas. (i.e., Pid = Pobs + constant). 2. Real gas molecules do not occupy an infinitely small volume (they are not point masses). At low pressures, the volume occupied by the gas molecules is much less than the container volume, and the gas molecules can essentially sample the entire container volume . However, when a gas is significantly compressed, the gas molecules themselves don’t decrease in size. Hence, the free space available to the gas molecules decreases. (Vid = Vobs - const.) The Van der Waal's Equation of State 18 Vid = Vobs - nb where b is a constant for specific to different gases. Pid = Pobs + a (n / V)2 where a is also different for different gases. Note that the pressure deviation is dependent on the concentration of the gas (n/V) squared. This is due to the fact that we are dealing with a twobody collision. gas Law Pid Vid = nRT (Pobs + a (n / V)2) x (Vobs - nb) = nRT This is the Van der Waals’s equation of state for real gases. It contains two constants (a, b) which must be experimentally determined for each individual gas. (see Table 10.3 in text). How do calculations with the ideal gas equation compare with those of the Van der Waal's equation? Example A sample of dry ice (CO2(s)) is placed in a heavy-walled evacuated container (V = 1.98L). After the dry ice has sublimed, the temperature of the system was measured to be 299.2K. Calculate the pressure of CO2 from the Van der Waal’s equation and the ideal gas equation. V = 1.98L a = 3.59 L2 atm / mole 2 T = 299.2K b = 0.0427 L / mole n = 215g CO2 x 1 mol CO2/44.01g = 4.89 mol CO2 19 Van der Waal’s equation 2 (Pobs n a x Vobs - nb = nRT V 2 L2 atm 4.89mol L (Pobs 3.59 x 1.98L 4.89molx0.0427 b . L mol mol 2 198 L atm = 4.89 molx0.08206 299.2 K K mol after solving for Pobs, we get Pobs = 45.9 atm. From the ideal gas equation PV nRT P nRT V Latm x299.2 K ) Kmol 198 . L ( 4.89 mol x 0.08206 P P=60.7 atm