Gas Laws: Boyle's, Charles's, Ideal Gas Law Explained
advertisement

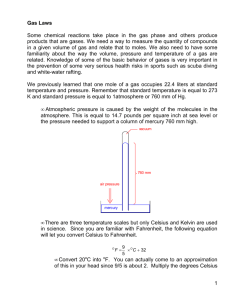
Unit 14: Gas Laws Pressure (P): The force of gas molecules as they hit the sides of the container in which they are placed. atmospheres (atm): The average air pressure at sea level. kilopascals (kPa): The SI unit for pressure; 101.325 kPa = 1 atm. mm Hg (Torr): 760 Torr = 1 atm. Volume (V): The amount of space in which a gas is enclosed in liters (L). Temperature (T): A measurement of the amount of energy that molecules have. The higher the energy, the higher the temperature. o Common units of temperature: Kelvin (K): The only units that can be used when doing numerical problems with gases. Degrees Celsius (0C): Must be converted to Kelvin before doing problems (by adding 273). STP: Stands for “standard temperature and pressure”, namely 273 K (00 C) and 1.00 atm. “Room temperature”: 298 K (250 C) Boyle’s Law: P1V1 = P2V2 Moles and temperature are being held constant o If I have 10 L of gas at a pressure of 1 atm and double the pressure, what will the new volume of the gas be? o If 250 L of a gas is in a sealed container at a pressure of 1.5 atm and I decrease the volume of the container to 100 L, what will the gas pressure inside the container be? Charles Law V1 V2 T1 T2 If you increase the temperature of a gas, the volume also increases. (Note: The temperature must be in Kelvin, NOT degrees centigrade) The KMT tells us that the amount of energy that a gas has is determined by the temperature of the gas. The more energy a gas has, the faster the gas molecules move away from each other, causing more space between the molecules and a larger overall volume. Sample problems o If you heat a 1.25 L balloon from a temperature of 250 C to 400 C, what will the new volume of the balloon be? o What temperature will be required to raise the volume of a 1.0 L balloon to 1.25 L if the initial temperature is 250 C? Combined Gas Law P PV 1V 1 2 2 T1 T2 o If one of the variables isn’t mentioned, we can assume that it’s kept constant and we can just cross it out of the equation. Sample problems o If I have 25 mL of a gas at a pressure of 2.1 atm and a temperature of 300 K, what will the pressure become if I raise the temperature to 400 K and decrease the volume to 10 mL? o If I have a container with an internal pressure of 1.5 atm and temperature of 250 C, what will the pressure be if I heat the container to 1500 C? Ideal Gases: Avogadro’s law: One mole of every gas has the same volume. This law assumes that all gases behave perfectly and identically according to the rules of the kinetic molecular theory. Though not precisely true, it gives us very good answers under most conditions. Ideal gas: A gas that behaves according to the kinetic molecular theory. o No intermolecular forces, infinitely small, etc. o There is no ideal gas in the real world, but some gases come closer than others: The gas molecules are small. The gas molecules have very weak intermolecular forces. The gas molecules are very hot, so they move quickly around and don’t interact with each other much. The gas is at low pressure, so the molecules have a lot of space between them. Ideal gas law: PV = nRT P = pressure (in atm of kPa) V = volume (L) n = number of moles T = temperature (Kelvin) R = ideal gas constant (depends on the unit of pressure used) 8.314 L kPa/mol K 0.08206 L atm/mol K Sample problems If I have 10 liters of a gas at a pressure of 1.5 atm and a temperature of 250 C, how many moles of gas do I have? If I have 3.5 moles of a gas at a pressure of 895 kPa and they take up a volume of 50 L, what’s the temperature? Gas Stoichiometry – Honors Chemistry At STP, one mole has a volume of 22.4 liters. If the compounds you’re working with on both sides of the equation are measured in liters, you can use the mole ratio conversion directly between them. Using the equation 2 H2 + O2 2 H2O, how many liters of water can be made from 25 liters of oxygen? If we do the reaction above using electrochemistry, we can make liquid water from H2 and O2 gas. If I use 20 L of oxygen gas at a pressure of 1.0 atm and a temperature of -350 C, how many grams of water will I make? Gas Stoichiometry – General Chemistry Explain to the general chemistry classes that at STP, the conversion factor between liters and moles is 22.4 L = 1 mole. What this means is that, whatever method of stoichiometry we use, we need only replace the molar masses of the gases with the number 22.4 …… for compounds that are solids, we still use the molar mass. Example: Using the equation 2 H2 + O2 2 H2O, how many liters of water can be made from 25 liters of oxygen at STP? Example : How many liters of water can be made from 240 grams of hydrogen?