Life of a Protocol - DAIDS Regulatory Support Center
advertisement

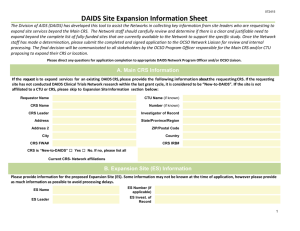
Standard DAIDS Protocol Development Steps by Role Protocol Development Step DAIDS CTA Initiation CTA initiated Protocol Team DAIDS RSC CTA development support initiated Team submits protocol to DAIDS for C/PSRC C/PSRC Review C/PSRC Review C/PSRC Review support Team submits revised protocol to DAIDS DAIDS approves protocol for Regulatory Review Regulatory Review Team submits protocol to RSC for Regulatory Review Regulatory Review and Processing Regulatory Review Team submits revised protocol to RSC for Medical Officer Review Medical Officer Review Medical Officer Review Processing Medical Officer Review Team submits revised protocol to RSC for Final RAB sign-off Final RAB Sign-off Final RAB sign-off Final RAB sign-off processing Protocol submitted to FDA and Ops for distribution to sites CTA finalized CTA finalization support IB distributed/PIs posted Sites can begin to submit materials to their IRB/EC and other regulatory agencies Regulatory Requirements Activities Protocol Registration If there are clinical hold issues, Team replies to FDA’s request for information (in most cases, there is not a clinical hold by the FDA, so protocol implementation can proceed) RSC notifies Team that there are no Regulatory holds Protocol Registration (Materials received and processed by PRO) Protocol Registration Study opens 1 July 8, 2013 DAIDS and DAIDS RSC Groups Involved in Protocol Development DAIDS: CTAT - The Clinical Trials Agreement Team (CTAT) negotiates CTAs and other research agreements between DAIDS and industry collaborators (study product manufacturers). C/PSRC - The Clinical/Prevention Science Review Committee (C/PSRC) is a reviewing body instituted by DAIDS to review Concept Sheets, Grants, Protocols, Sub-studies, and Amendments to Protocols developed by various programs seeking DAIDS support. The C/PSRC reviews each proposal to assess its scientific merit, plans to ensure volunteer safety, and compliance with ethical and regulatory requirements. Support of the clinical research proposal is weighed in relation to the National Institute of Allergy and Infectious Diseases (NIAID) HIV AIDS scientific priorities, and other planned or ongoing clinical studies. Prior to the implementation of any proposal, C/PSRC approval must be obtained. The RSC provides support for these activities with the distribution of documents for review, agendas for meetings, preparation of Consensus Memos reflecting the Committees’ comments, and distribution of the Committees’ responses to protocol teams. MO - The Medical Officer (MO) is a DAIDS staff member or member from another sponsoring Institute or Centers that monitors the safety and efficacy of the intervention(s) for ongoing studies and those in development. ProPEP - The Protection of Participants, Evaluation, and Policy Branch (ProPEP) provides guidance for the DAIDS RSC Human Subjects Protection team’s reviews of informed consents and Spanish Translations. PRT - The Protocol Registration Team (PRT) within the Office of Clinical Research Policy and Resources (OPCRO) responsible for managing the Protocol Registration System, which includes oversight of the DAIDS PRO. RAB - The Regulatory Affairs Branch (RAB) within DAIDS is part of OPCRO. RAB performs regulatory surveillance over clinical trials sponsored/funded by DAIDS. The DAIDS Regulatory Support Center (RSC): DAIDS RSC CTA - The Clinical Trials Agreement (CTA) team supports and facilitates the negotiation of CTAs and other research agreements between DAIDS and industry collaborators (study product manufacturers). DAIDS RSC HSP - The Human Subjects Protection (HSP) team is responsible for reviewing all Informed Consents (ICs) during review at the C/PSRC, Regulatory Review, and Protocol Registration. These include ICs for DAIDS-sponsored network and investigator-initiated protocols supported through DAIDS’ grants. The HSP team is responsible for the translation of Sample ICs to Spanish. DAIDS RSC Regulatory - The Regulatory Team reviews Protocol Documents for regulatory compliance, and prepares and files new Investigational New Drug Applications (INDs) and amendments to existing INDs in compliance with the procedural and substantive requirements of 21 CFR § 312. Examples of submissions to the FDA include original IND Applications, Annual Reports, Safety Reports, and Responses to FDA Requests for Information. 2 July 8, 2013 DAIDS RSC PRO - The Protocol Registration Office (PRO) registers and de-registers sites that will be running studies and enrolling participants. This involves collecting and processing ICs, Institutional Review Board (IRB)/Ethics Committee (EC) approval letters, Food and Drug Administration (FDA) 1572 Forms, Investigator of Record (IoR) forms, and the Principal Investigator’s curriculum vitae. Protocol registration may occur more than once during the course of the protocol. Subsequent protocol registrations are called amendment registrations. DAIDS RSC RIC - The Regulatory Support Center Safety Information Center (RIC) distributes Investigators’ Brochures (IBs), Package Inserts (PIs), Safety Reports, Data Safety Monitoring Board (DSMB) Reports, and Risk Lists to the FDA, Study Coordinators, drug companies, and DAIDS. 3 July 8, 2013