GCLP Standards - DAIDS Learning Portal
advertisement

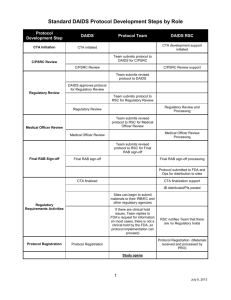
Introduction to Good Clinical Laboratory Practices (GCLP) Version 5.0, August 2012 This project has been funded in whole or in part with Federal funds from the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN272201200009C, entitled NIAID HIV and Other Infectious Diseases Clinical Research Support Services (CRSS). Workshop Approach Objectives Didactic Presentation Activities and Discussion 2 Workshop Materials DAIDS GCLP Standards Booklet Module Handouts Reference Materials ARS Key Pads 3 Audience Response System (ARS) 4 Respond to Questions Change an Answer Responses are Anonymous Question cue is a on preceding slide Ensure remote is on by pressing and holding the “On/Off” button Please leave remotes on the tables Choose your answer Send or change your answer Audience Response System (ARS) (con’t) Good Documentation Practices include following the principles of: A. Electronic Record Management B. “ALCOA” C. Electronic Signatures Choose your answer Send or change your answer 5 Audience Response System (ARS) (con’t) Name one of the words that is applied within the acronym “ALCOA.” Choose your answer Send or change your answer 6 Survey What position do you hold in your facility? A. Laboratory Technician B. QA/QC Coordinator C. Laboratory Manager D. Laboratory Director E. Other (laboratory) F. Other (nonlaboratory) 7 Survey (con’t) How many years of research experience do you have? A. None B. < 1 C. 1 – 5 D. 6 – 10 E. >10 8 Objectives 9 Explain what GCLP is and why it is important List GCLP standards List the key entities involved in ensuring GCLP Explain the role that audits play in ensuring GCLP Pre-Assessment Question #1 Good Clinical Laboratory Practices are important for which of the following reasons? A. To ensure accurate laboratory testing results B. To allow for reconstruction of the study C. To define the performance of specific laboratory functions D. A and B only E. A, B and C 10 Pre-Assessment Question #2 According to GCLP, accurate documentation is important for which of the following reasons? A. To allow for reconstruction of a study B. To ensure that data are reliable, and accurately recorded C. To explain why, how, and by whom an activity was performed D. All of the above 11 Pre-Assessment Question #3 Which organization generates action plan documents after international audits and supports Corrective Action and Preventive Action (CAPA), External Quality Assurance (EQA) / Proficiency Testing (PT)? A. DAIDS B. Patient Safety Monitoring in International Laboratories (SMILE) C. DAIDS Clinical Laboratory Oversight Team (DCLOT) D. CRSS 12 Pre-Assessment Question #4 Site monitors ensure compliance to GCLP standards by conducting __________________. A. Site visits B. Data entry C. Laboratory audits D. A and B only E. A and C only F. None of the above 13 Laboratory Oversight Relationships DAIDS DCLOT SMILE CRSS WESTAT FHI DCLOT and SMILE generate action plan documents after audits; -SMILE supports CAPA, EQA/PT. 14 Clinical Operations Clinical Support - Oversee Laboratory Audits LABORATORIES & REPOSITORIES DAIDS Guidelines for GCLP Standards 15 Outlines the minimal requirements clinical research laboratories should follow Encompass elements of: GLP (21CFR Part 58), CLIA (42CFR Part 493), and Guidance from ISO and CAP Deployed globally Compliance is an ongoing process Monitoring of compliance by the DAIDS through site visits and laboratory audits GCLP Standards Provide for… Sufficiently trained, experienced, and competent staff Appropriate and adequate facilities Optimal functioning equipment Adequate reagents and materials Relevant standard operating procedures (SOPs) that are approved and in place for use by laboratory personnel And ensure that… Analytical Plans exist Quality Assurance and Quality Control programs are in place Specimen integrity is maintained 16 Why GCLP? To ensure quality, reliability, and integrity of study data To ensure comparability of study data To allow for reconstruction of the study To achieve uniformity of the performance of specific functions across all laboratories worldwide 17 Elements of GCLP Organization and Personnel Safety Facilities and Equipment Verification of Performance Specifications Laboratory Information Systems (LIS) Testing Facilities Operation Test and Control Articles Records and Reports Specimen Management and Tracking Quality Management 18 Documentation “If it is not documented, it never happened!” Maintenance of prompt, accurate, and complete records Why, how, when, and by whom was the test performed “Write down what you do, do what is written down!” 19 Study Data FDA expects data acquired during a clinical study to be: A L C O A 20 ttributable egible ontemporaneous riginal ccurate GCLP Standards Compliance to GCLP standards will ensure that data reported is: Consistent Reproducible Auditable Produced in an environment conducive to study reconstruction 21 Additional Training Resources DAIDS Learning Management System (DLMS): https://daidslms.plateau.com/learning/user/login.do Laboratory Curriculum GCLP Online Review Courses Quality Management Curriculum CAPA for Laboratory Staff FSTRF Training Laboratory Data Management System (LDMS) Courses 22 Comprehensive GCLP Workshop DAIDS Laboratory Audits General Laboratory Audit Central Laboratory Audit Histology Audit Types of Audits PBMC Laboratory Audit Repository Audit TB Laboratory Audit 23 DAIDS-Sponsored Programs GCLP Standards GCLP Training 24 GCLP Audits Skill Check A DAIDS audit has just been announced in your laboratory. How should your laboratory respond? A. The QA/QC Coordinator should sign and predate all temperature charts that were not reviewed the previous year B. Send all staff without complete personnel records on forced vacation C. Embrace the audit and use the opportunity to improve your systems D. Move all equipment without documented maintenance out of the laboratory and bring it back as soon as the auditor has left E. None of the above 25 Teamwork Teamwork is the ability to work together toward a common vision—the ability to direct individual accomplishments toward organizational objectives. It is the fuel that allows common people to attain uncommon results. Andrew Carnegie 26 Post-Assessment Question #1 Good Clinical Laboratory Practices are important for which of the following reasons: A. To ensure accurate laboratory testing results B. To allow for reconstruction of the study C. To define the performance of specific laboratory functions D. A and B only E. A, B and C 27 Post-Assessment Question #2 According to GCLP, accurate documentation is important for which of the following reasons? A. To allow for reconstruction of a study B. To ensure that data are reliable, and accurately recorded C. To explain why, how, and by whom an activity was performed D. All of the above 28 Post-Assessment Question #3 Which organization generates action plan documents after international audits and supports CAPA, EQA/PT? A. DAIDS B. SMILE C. DCLOT D. CRSS 29 Post-Assessment Question #4 Site monitors ensure compliance to GCLP standards by conducting __________________. A. Site visits B. Data entry C. Laboratory audits D. A and B only E. A and C only F. None of the above 30 Wrap Up 31