Functional Groups - Chemistry Courses: About
advertisement

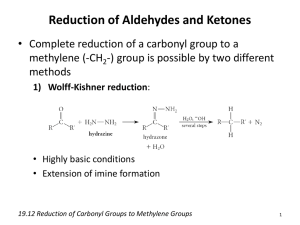
Functional groups One of the organizing principles of organic chemistry is the “functional group.” While it might seem that there are infinite possibilities o f how atoms might be connected to each other, it turns out that there are a small number of typical patterns. Why is this the case? First, we primarily work with only four elements: hydrogen, carbon, nitrogen, and oxygen. Second, remember the bonding rules we have for typical, stable atoms in a molecule—carbon forms four bonds, nitrogen forms three bonds and has a lone pair, and oxygen forms two bonds and has two lone pairs. With these limitations, there are only a few patterns that we need to recognize, and we call each pattern of bonding a functional group and give it its own name. Below are the major functional groups that we will study. It is essential that you have the following skills: Be able to recognize what distinguishes each functional group from the others. Be able to recognize the functional group within the context of a larger molecule. Be able to name the functional group Know and be able to use a few other terms that are related to each functional group 1. Alkane The “alkane” is the most basic unit in organic chemistry, made up of carbon/carbon single bonds and carbon/hydrogen single bonds. In fact, we often don’t consider the alkane to be a functional group at all, but rather the molecular scaffold on which functional groups are placed. Think of the alkane as the canvas on which a picture is painted. We can use the term “alkane” to refer to a whole molecule that contains nothing but carbon and hydrogen single bonds, or we can refer to a portion of a molecule as the alkane portion of the molecule. Alkanes are a type of hydrocarbon, a molecule that contains only carbon and hydrogen. Some other important common terms associated with alkanes include straight-chain alkane, branched alkane, and cyclic alkane. Examples of alkanes: cyclic straight-chain branched The molecule below is cholesterol. We might say that the molecule has an “alkane portion” which is circled. An older term that is still common is to say that cholesterol contains an “aliphatic portion” because it is all carbon and hydrogen single bonds. HO 2. Alkenes The alkene is also a hydrocarbon because it contains only carbon and hydrogen, but it also contains a carbon-carbon double bond. We say that the alkene is unsaturated because it is not “saturated with hydrogen atoms—it does not contain as many C-H bonds as possible. The base unit of the alkene is the C=C, with hydrogen atoms or “R” groups attached. The “R” group is the generic variable of any alkane group. We refer to this as an “alkyl group. R or H R or H C base unit of an alkene C R or H R or H We might use the term alkene in two ways: we might refer to the C=C bond itself as an alkene, or we might refer to a whole molecule containing the carbon-carbon double bond as an alkene. For example, we might say that there are three alkenes shown below, or we might say that the three molecules below all contain the alkene functional group. Because of their structural similarities, all alkenes have some similarities in their properties and reactivity. a cyclic alkene 3. Alkyne The alkyne is similar to the alkene except that alkynes contain carbon-carbon triple bonds. They are also unsaturated hydrocarbons. A terminal alkene has at least one hydrogen directly attached to the triple bonded carbon. An internal alkene has two R groups. The prime symbol in R’ designates that this R group may be different than the other R group. R C C terminal alkyne 4. Aromatic H R C C internal alkyne R' In this course, we will learn quite a bit about aromatic compounds. For now, we will discuss the most common aromatic structure: benzene. Benzene is a six member carbon ring with three double bonds. benzene aromatic compounds 5. Alcohol An alcohol is a molecule that contains the hydroxyl group, -OH. Therefore, the generic representation of an alcohol is ROH. The base unit is carbon single bonded to oxygen, which is single bonded to hydrogen. R or H R' or H C O H OH OH R' or H base unit of alcohol moleucle A molecule B The two example molecules above both contain the base unit C-O-H. All of the following statements would be proper to make: Molecules A and B are alcohols. Molecule A contains a hydroxyl group. Molecule B is an alkene. Molecule B is a cyclic molecule containing a hydroxyl group. 6. Phenol Alcohols that are directly connected to benzene have a special name: phenol. It is important to recognize that the phenol involves the hydroxyl directly connected to the benzene, not simply in the same molecule. OH OH a phenol 5. Ether not a phenol--an alcohol The ether is a functional group similar to the alcohol, but there is one crucial distinction: the oxygen atom is bonded to two carbon atoms. Therefore the base unit of the ether is C-O-C. O O a symmetrical ether O a cyclic ether an unsaturated ether 6. Amine The distinctive of the amine group is that it contains a nitrogen atom bonded to hydrogen and/or alkyl groups. Amines can look quite different, as in the examples below. H N H2N N N H 7. Nitrile A nitrile contains the cyano functional group, which is a carbon-nitrogen triple bond. Due to the nature of nitrogen bonding, the cyano group is always a terminal group in a compound. It is often written in its condensed formula, “CN”. Therefore, the general representation of a nitrile is RCN. CN N an aromatic nitrile an aliphatic nitrile 7. Thiol Thiols are the equivalent of alcohols that have sulfur instead of oxygen. Therefore, the base unit of a thiol is C-S-H and the general designation for a thiol is RSH. 8. Alkyl halide The alkyl halide is a alkane skeleton with a halogen bonded. The halogens (fluorine, chlorine, bromine, and iodine) can be represented generically as “X”, and therefore, the general alkyl halide can be designated RX. Cl I Br 9. Ketone The ketone is the first of many compounds that contains the carbonyl group, which is a carbonoxygen double bond. A compound is a ketone if the carbonyl carbon is directly attached to two carbon atoms, typically alkanes or alkenes. O O C C O C base unit of ketone cyclic keton an unsaturated ketone 10. Aldehyde An aldehyde is very similar to a ketone, but in an aldehyde, the carbonyl is attached to carbon on one side and hydrogen on the other. Therefore, the aldehyde is always at the end of a molecule. In the condensed formula, the aldehyde is designated with “CHO” which does not mean that hydrogen is single bonded to carbon and oxygen, but is meant to distinguish the group from a hydroxyl group. The general designation of an aldehyde is RCHO. O O C C H base unit of aldehyde CH3CH2CHO a condensed formula of an aldehyde H an aromatic aldehyde 11. Carboxylic acid A carboxylic acid contains a carbonyl attached to an R group on one side and a hydroxyl group on the other side. NOTICE: The carboxylic acid is NOT a ketone and alcohol, but rather, a whole new group. Therefore it is proper to say that all carboxylic acids contain a hydroxyl group, but not all hydroxyl groups are carboxylic acids. Condensed formula for a carboxylic acid is COOH, and these acids are generally written RCOOH. Two carboxylic acids in the Citric Acid Cycle O C C succinate fumarate OH COOH base unit of aldehyde COOH HOOC HOOC an unsaturated carboxylic acid 12. Ester The ester contains a carbonyl attached to an R group on one side and an OR group on the other side. O O C C O OR O O base unit of ester 13. Amide An amide contains a carbonyl with an R group on one side and a nitrogen atom on the other. The nitrogen may be attached to other R groups or hydrogens. NOTICE: The amide is NOT a ketone attached to an amine, but a whole new group called an amide. O O O H2N C C N base unit of ester HN N H O