SKILL #3 MOLAR MASS OF A COMPOUND
advertisement
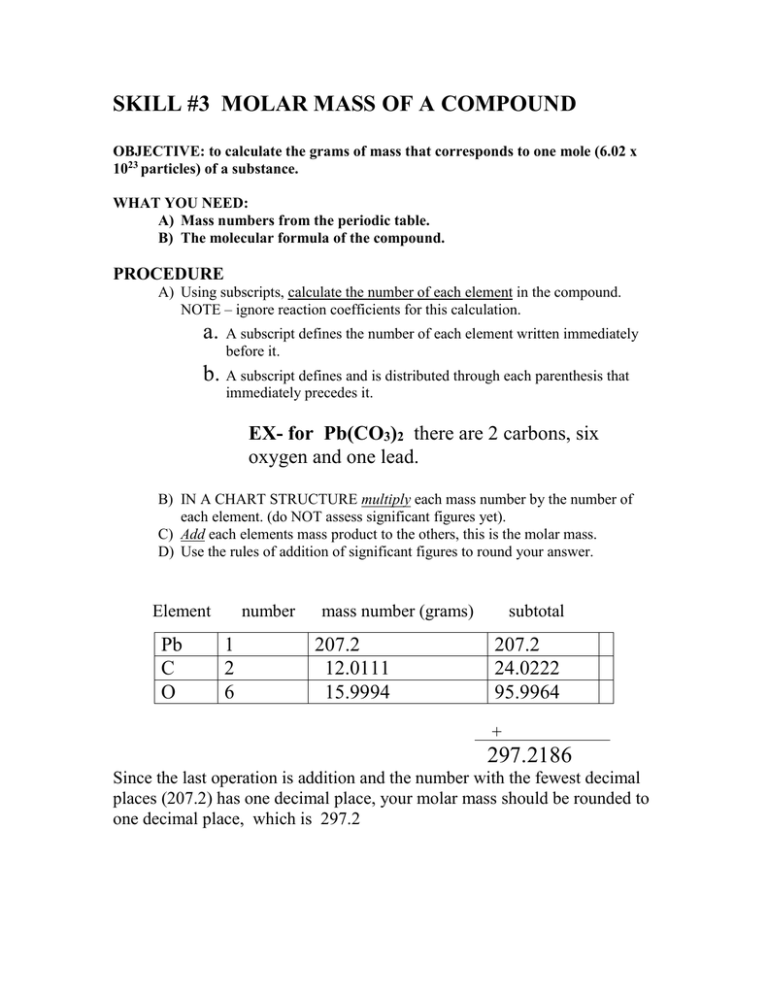
SKILL #3 MOLAR MASS OF A COMPOUND OBJECTIVE: to calculate the grams of mass that corresponds to one mole (6.02 x 1023 particles) of a substance. WHAT YOU NEED: A) Mass numbers from the periodic table. B) The molecular formula of the compound. PROCEDURE A) Using subscripts, calculate the number of each element in the compound. NOTE – ignore reaction coefficients for this calculation. a. A subscript defines the number of each element written immediately before it. b. A subscript defines and is distributed through each parenthesis that immediately precedes it. EX- for Pb(CO3)2 there are 2 carbons, six oxygen and one lead. B) IN A CHART STRUCTURE multiply each mass number by the number of each element. (do NOT assess significant figures yet). C) Add each elements mass product to the others, this is the molar mass. D) Use the rules of addition of significant figures to round your answer. Element Pb C O number 1 2 6 mass number (grams) 207.2 12.0111 15.9994 subtotal 207.2 24.0222 95.9964 + 297.2186 Since the last operation is addition and the number with the fewest decimal places (207.2) has one decimal place, your molar mass should be rounded to one decimal place, which is 297.2