CTN - Austin Health
advertisement

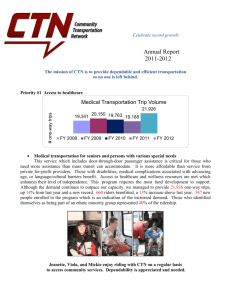
Austin Health Clinical Trials Notification form guidelines Do I need a CTN for my clinical research? If your project includes one of the following then a Clinical Trial Notification (CTN) form must be completed and must be included in your Ethics & Governance submission: Any product not approved by the Therapeutic Goods Administration (TGA). This could be a drug, therapeutic device or even diagnostic machines. Use of an approved product in a clinical trial beyond the conditions of its approval (i.e. in an unapproved indication, age group, dosage, administration route) How do I Complete a CTN Form? An original CTN (1 only and preferably on blue paper) must be submitted with the ethics submission. If the study is multi-site you will need to submit a CTN for each site to the reviewing HREC. Please ensure all questions on the form are complete before submission to the Ethics Office. The CTN can be submitted to Ethics & Research with no signatures. Please note that all unregistered therapeutic goods need to be listed in the CTN, this includes a placebo. Trial site details for a study being conducted at Austin Health Site: Austin Health Site Address: 145 Studley Rd Heidelberg, 3084 HREC details for a study being reviewed by Austin Health HREC HREC name: Austin Health Human Research Ethics Committee HREC address: 145 Studley Rd Heidelberg 3084 Name (Print): Prof David Taylor Position: HREC Chairperson Phone: 03 9496 4711 Fax: 03 9496 4103 Authority approving details for studies being conducted at Austin Health Approving Authority name: Austin Health Address: 145 Studley Rd Heidelberg 3084 Name (Print): Dr Sianna Panagiotopoulos Position: Manager, Office for Research Phone: 03 9496 5088 Fax: 03 9496 4103 Who is the sponsor? The sponsor of a clinical trial can be: An individual (eg medical practitioners) An organisation (eg hospital, Area Health Service, non-government organisations) or A Company (eg pharmaceutical companies) The sponsor is responsible for the overall conduct of the study. I have ethics and governance approval what’s next? Once the sponsor, the principal investigator, the Chairman of the HREC and the person responsible from the Approving Authority have signed the CTN Form, it is submitted to the TGA with the notification fee. Please visit the TGA website for further details. CTN guidelines Version 2 14 March 2013 Investigator Initiated Studies If the study is Investigator Initiated Austin Health will usually sign off on the CTN as the sponsor. It is the responsibility of the Investigator to submit the completed CTN to the TGA prior to study commencement. The Investigator is also responsible for organising and submitting the CTN trials completion form. CTN faux pas The CTN may be rejected by the TGA if submitted with any of the following: Electronic signatures, all signatures on the CTN must be original wet ink signatures. Liquid paper is not acceptable on any part of the CTN. Any section of the document is not completed, signed, dated or the signees’ details have not been inserted. If the start date listed on the CTN is well before the Approving Authority signature date. For further information on CTNs and to access the most up to date version of the form please visit the TGA website http://www.tga.gov.au/industry/clinical-trials.htm\ CTN guidelines Version 2 14 March 2013