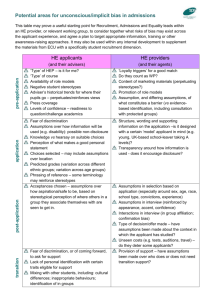
Internal Comms - Calvary Health Care Bruce
advertisement
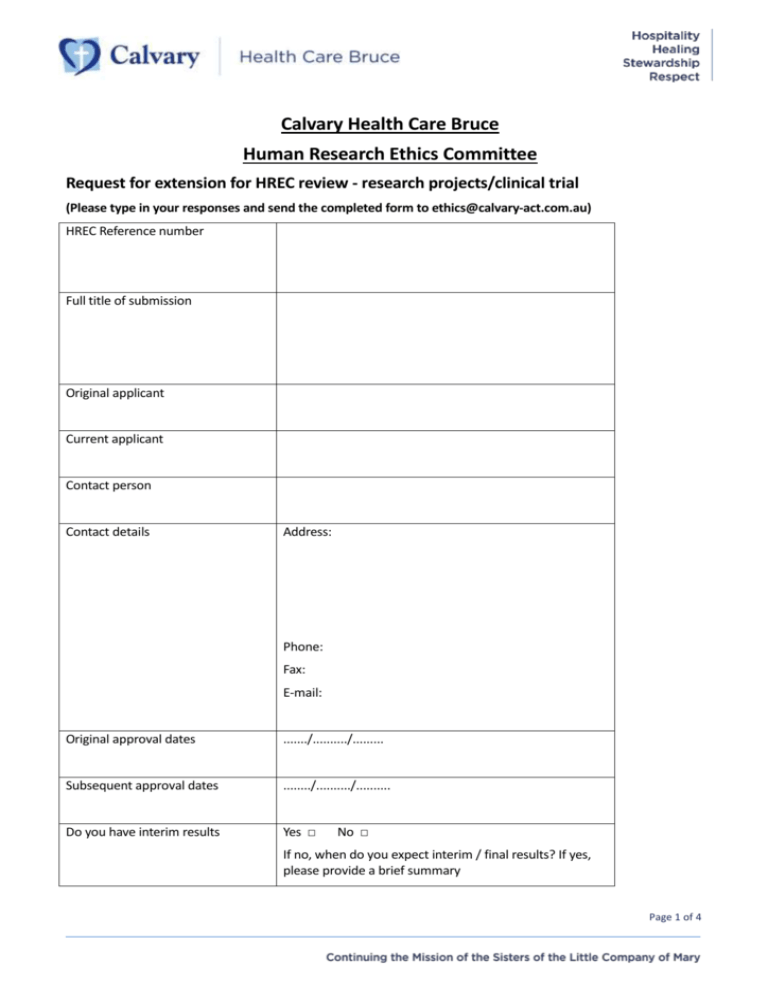
Calvary Health Care Bruce Human Research Ethics Committee Request for extension for HREC review - research projects/clinical trial (Please type in your responses and send the completed form to ethics@calvary-act.com.au) HREC Reference number Full title of submission Original applicant Current applicant Contact person Contact details Address: Phone: Fax: E-mail: Original approval dates ......./........../......... Subsequent approval dates ......../........../.......... Do you have interim results Yes □ No □ If no, when do you expect interim / final results? If yes, please provide a brief summary Page 1 of 4 Is the project being conducted in accordance with the approved protocol Yes □ No □ Does the project involve participant recruitment Yes □ No □ Have there been significant events that impact on the study (SAEs, SUSARs) in the past 12 months Yes □ No □ Summarise changes Have findings of ethical significance arisen at this site or elsewhere Yes □ No □ Is recruitment to continue If Yes, please explain: Yes □ Have participants been informed of these events Yes □ If yes, please provide a brief summary If No, detail the changes: No □ N/A □ No □ Page 2 of 4 Current request extension dates ........./.........../.......... Can you explain in detail the reasons for extension Are there changes envisaged to: Study personnel at Calvary HCACT Yes □ No □ If yes, please provide brief summary Quality control Yes □ No □ Monitoring /audit of project Yes □ No □ Date informed: ...../..../..... State changes: Page 3 of 4 Insurance Yes □ No □ Compensation Yes □ State changes: No □ Supporting documents (brochures, survey questionnaire etc) Yes □ State changes: If yes, please provide the clean and tracked versions of the same No □ New aims and / or substantial changes in the protocol Yes □ No □ For the single-site studies - In the continued research, will it develop into a multi-site study? Yes □ No □ Have you submitted all progress and annual reports so far Yes □ No □ If yes, please provide a brief summary and a clean and tracked versions of the documents If yes, name the sites and mention if ethics approval has been granted to undertake research in these sites Applicant Signature Applicant Name (print) Date ......./........../.......... Page 4 of 4