Formulas and moles
advertisement

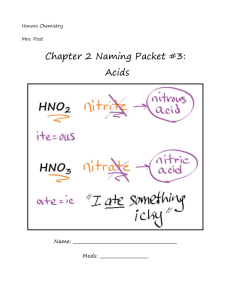
Naming Molecular (Covalent) Compounds We also need to take up the matter of naming covalent compounds. Covalent compounds are named in different ways than are ionic compounds (although there is some overlap). Many of these compounds have common names such as "methane", "ammonia" and "water". However, simple covalent compounds are generally named by using prefixes to indicate how many atoms of each element are shown in the formula. Also, the ending of the last (most negative) element is changed to -ide. The prefixes used are mono-, di-, tri-, tetra-, penta-, hexa-, and so forth. The mono- prefix is usually not used for the first element in the formula. The "o" and "a" endings of these prefixes are dropped when they are attached to "oxide." 1 mono- 2 di- 3 tri- 4 tetra- 5 penta- 6 hexa- You also need to know which element to put first in the formulas and names of these compounds. Generally, they are in the same left-to-right order that they have on the periodic table, except that you would have to squeeze hydrogen in between nitrogen and oxygen. At this time, try the following practice problems (also found in exercise 12 in the workbook). Name the following compounds. PH3 CO HI N2O3 SCl6 P2O5 SO3 (notice: no charge) NH3 Naming Ionic Compounds Name the cation (Determine the charge of the transition metal if more than one option is available- AND use that in the name) and then name the anion. Give the name of the following ionic compounds: 1) Na2CO3 ____________________________________________________ 2) NaOH _____________________________________________________ 3) MgBr2 _____________________________________________________ 4) KCl _______________________________________________________ 5) FeCl2 ______________________________________________________ 6) FeCl3 ______________________________________________________ 7) Zn(OH)2 ___________________________________________________ 8) BeSO4 ___________________________________________________ 9) CrF2 ______________________________________________________ 10) Al2S3 _____________________________________________________ 11) PbO ______________________________________________________ 12) Li3PO4 ____________________________________________________ 13) TiI4 _______________________________________________________ 14) Co3N2 ____________________________________________________ 15) Mg3P2 ____________________________________________________ 16) Ga(NO2)3 __________________________________________________ 17) Ag2SO3 ____________________________________________________ 18) NH4OH ____________________________________________________ 19) Al(CN)3 ____________________________________________________ 20) Be(C2H3O2)2 ______________________________________________ Writing Ionic Formulas For the following compounds, give the formulas 22) sodium phosphide ___________________________________________ 23) magnesium nitrate ___________________________________________ 24) lead (II) sulfite ______________________________________________ 25) calcium phosphate ___________________________________________ 26) ammonium sulfate ___________________________________________ 27) silver cyanide _______________________________________________ 28) aluminum sulfide ____________________________________________ 29) beryllium chloride ____________________________________________ 30) copper (I) arsenide ___________________________________________ 31) iron (III) oxide _______________________________________________ 32) gallium nitride _______________________________________________ 33) iron (II) bromide _____________________________________________ 34) vanadium (V) phosphate ______________________________________ 35) calcium oxide _______________________________________________ 36) magnesium acetate __________________________________________ 37) aluminum sulfate ____________________________________________ 38) copper (I) carbonate __________________________________________ 39) barium oxide ________________________________________________ 40) ammonium sulfite ____________________________________________ 41) silver bromide _______________________________________________ 42) lead (IV) nitrite ______________________________________________ Rules for Naming Simple Acids and Oxyacids How do you recognize that something is an acid? The acids that we will be concerned with naming are really just a special class of ionic compounds where the cation is always H+. By convention, cations are written first in ionic formulas. So if the formula has hydrogen written first, then this usually indicates that the hydrogen is an H+ cation and that the compound is an acid. When dissolved in water, acids produce H+ ions (also called protons, since removing the single electron from a neutral hydrogen atom leaves behind one proton). If the counterion (the anion) to H+ in the acid is a polyatomic ion that contains oxygen (like NO2- or PO43-), the acid is called an oxyacid and is named using the rules provided below. If the anion does not contain oxygen, as in the case of F- or the polyatomic ion CN-, then a different set of rules are used for naming the acid. Rules for Naming Oxyacids (anion contains the element oxygen): Since all these acids have the same cation, H+, we don't need to name the cation. The acid name comes from the root name of the oxyanion name or the central element of the oxyanion. Suffixes are used based on the ending of the original name of the oxyanion. If the name of the polyatomic anion ended with -ate, change it to -ic for the acid and if it ended with -ite, change it to -ous in the acid. -ate becomes –ic and -ite becomes -ous So, HNO3, which contains the polyatomic ion nitrate, is called nitric acid. The acid HNO2, which contains the polyatomic ion nitrite, is called nitrous acid. Rules for Naming Acids that Do Not Contain Oxygen in the Anion: Since all these acids have the same cation, H+, we don't need to name the cation. The acid name comes from the root name of the anion name. The prefix hydro- and the suffix -ic are then added to the root name of the anion. So, HCl, which contains the anion chloride, is called hydrochloric acid. HCN, which contains the polyatomic ion cyanide, is called hydrocyanic acid. Name the following acids 1. HCl 2. HClO4 3. HIO3 4. HI 5. H2SO4 6. H2S 7. HCN 8. H2CO3 9. HBrO4 10. HBrO3 11. HC2H3O2 12. H3PO3 14. H2CrO4 15. H2Cr2O7 Give formulas for the following acids 17. hydrofluoric acid 18. nitric acid 19. nitrous acid 21. periodic acid 22. carbonic acid 23. hypobromous acid 24. bromous acid 25. permanganic acid 27. iodous acid 28. sulfurous acid 29. sulfuric acid 30. perchloric acid 31. chlorous acid 32. acetic acid 33. phosphoric acid 34. phosphorous acid Molecular Mass and Percent Composition Worksheet Find the molecular mass of each of the following to two decimal places AND use appropriate units. ex: H20: 2(1.01) + 1(16.00) = 18.02 grams/mole (**note the unit) 1. CO 2. CO2 3. CeBr3 4. H2SO4 5. CuSO4 6. H2CO3 7. MgSO4 8. C6H12O6 9. C2H3O 10. C2H5OH Determine the percent composition of each element in the following compounds. Express answers to two decimal places. ex: SO2 SO2 = 32.07 + 32.00 = 64.07 % S = mass S/mass SO2 X 100; 32.066/64.064 X 100 = 50.053% = 50.05% % O = mass O/mass SO2 X 100; 31.998/64.064 X 100 = 49.946 % = 49.95% 11. CO2 12. CH2O 13. H2SO4 14. CuSO4 15. CHCl3 16. C12H22O11 17. C14H20N2SO4 The Mole Is What Brings it Together 1 mole = 6.02 x 1023 molecules = 22.4 L (@ STP) 1. Calculate the mass of 1.58 moles CH4. [molar mass CH4 = 16.0 g/mol] 1.58 moles CH4 2. What volume will 7.29 moles of CO2 gas occupy at STP? 7.29 moles CO2 3. = = How many molecules are there in a 0.00583 mole sample of H2O? 0.00583 moles H2O = 4. What mass of CO2 gas occupies a volume of 100. Liters at STP? [molar mass CO2 = 44.0 g/mol] 5. How many molecules are in a 35.0 gram sample of H2O? [molar mass H2O = 18.0 g/mol] 6. What volume will 5.25 x 1022 molecules of CH4 occupy at STP? 7. What volume will 2.22 moles of CO2 gas occupy at STP? 8. How many molecules are there in a 0.127 mole sample of H2O? 9. What mass of CO2 gas occupies a volume of 395 Liters at STP? [molar mass CO2 = 44.0 g/mol] 10. Given 28.08 grams of C4H10 a. How many moles of butane molecules are present? b. How many water molecules are present? c. How many moles of C atoms are present? How many C atoms? d. How many moles of H atoms are present? How many H atoms?