Writing and Naming Acids
MS. GROBSKY
Acids and Their Formulas
Acids are an important class of hydrogen-containing compounds,
and they are named in a special way
A simple definition of an acid is a substance which produces H+
ions in water
Note – In order for a substance to be an acid instead of a gas (i.e. HCl),
binary acids must be aqueous or dissolved in water
Ternary (or oxy-) acids are ALWAYS aqueous
When we encounter the chemical formula for an acid, it will be
written with an H as the first element
Examples:
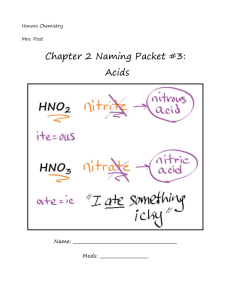
HCl (aq) – hydrochloric acid
HNO3 (aq)– nitric acid
H2SO4 (aq) – sulfuric acid
Naming Binary Acids
The name of the acid is related to the name of its anion
Acids containing anions without oxygen commonly end
in –ide
Called binary acids
These acids are named by:
3
Adding prefix hydro to the anion root
Changing the –ide ending of the anion to –ic
Adding the word acid after the anion
Examples:
HCl
HCN
H 2S
Hydrochloric acid
Hydrocyanic acid
Hydrosulfuric acid
Copyright © Cengage Learning. All
rights reserved
Binary Acids That Do Not Contain Oxygen
Naming Ternary Acids or Oxy-Acids
An oxy-acid consists of a hydrogen ion bonded to a
polyatomic ion that contains oxygen
These anions commonly end in –ate or –ite
These are more difficult to name because these acids
have hydrogen, a non-metal, and may have varying
number of oxygen atoms
For example, H2SO4 (aq), H2SO3 (aq), H2SO2 (aq) are all acids
So, how do we name them?!
To begin, we need a point of reference…
Rules for Naming Oxy-Acids
Acids containing anions whose names end in –ate are
named by:
Changing –ate to –ic
Note: Prefixes in the anion name are retained in the name of
the acid
Adding the word acid after the anion
If the acid has one more oxygen than the –ic acid,
use the prefix per- before the anion root name
Examples:
HClO4
Perchloric acid
HClO3
Chloric acid
HC2H3O2 Acetic acid
Rules for Naming Oxy-Acids
Acids containing anions whose names end in –ite are
named by:
Changing –ite to –ous
Note: Prefixes in the anion name are retained in the
name of the acid
Adding the word acid after the anion
If the acid has one less oxygen than the –ous acid,
use the prefix hypo- before the anion root name
Examples:
HClO2
HClO
H2SO3
Chlorous acid
Hypochlorous acid
Sulfurous acid
Some Oxygen-Containing Acids
Flowchart for Naming Acids
Anion Ends in -ide
Anion Ends in –ite or -ate
Practice!
• HBr (aq)
• No oxygen, -ide
hydrobromic acid
carbonic acid
nitrous acid
• H2CO3
• Has oxygen, -ate
• HNO2
• Has oxygen, -ite
Writing Chemical Formulas for Acids
An acid is composed of an anion connected to enough H+
ions to neutralize, or balance, the anion’s charge
You must work backwards from the name to figure out
the anion formula and charge!
Hydro-______-ic acid
_____-ic acid
Anion ends in –ide
Can be element from Periodic Table, cyanide, or hydroxide
Anion ends in –ate
Polyatomic anion from Pink Sheet!
_____-ous acid
Anion ends in –ite
Polyatomic anion from Pink Sheet!
Then, criss-cross charges from H+ and the anion!
Practice!
H3PO3
HI
H2C2O4
Hydrofluoric acid
Bromous acid
Phosphoric acid
12
Copyright © Cengage Learning. All
rights reserved
Naming and Writing Covalent
Chemical Formulas
What are Covalent Compounds?
Remember, ionic
compounds generally
involve a metal cation (or
ammonium) and a nonmetal anion (or
polyatomic anion) being
held together by an
electrostatic attraction
Compounds are called
formula units
Covalent compounds
consist of two nonmetals
sharing electrons
Compounds are called
molecules
Writing Covalent Compound Formulas
You never use “criss-
cross” method
Molecules share electrons
and therefore, do not have
full charges that need to
cancel!
Instead, the prefixes in
the name indicate the
number of atoms present
The prefix mono- is only
used if there is one of the
SECOND non-metal
Number
Prefix
1
Mono
2
Di
3
Tri
4
Tetra
5
Penta
6
Hexa
7
Hepta
8
Octa
9
Nona
10
Deca
Practice!
Phosphorus Trioxide
Dinitrogen Pentacarbide
Tellurium Noniodide
Carbon Monoxide
Selenium Heptaflouride
Tetraphosphorous
Decoxide
Arsenic Hexabromide
Silicon Dichloride
PO3
N 2C 5
TeI9
CO
SeF7
P4O10
AsBr6
SiCl2
Naming Covalent Compounds
The 1st nonmetal is just given the name as found on the
Periodic Table (just like ionic)
The 2nd nonmetal ending is changed to –ide (just like
ionic)
Difference: numerical prefixes are used to express how
many of each nonmetal are present
Exception: “mono” is not used in front of FIRST element’s name
CO2 Carbon dioxide
CO Carbon monoxide
PCl3 Phosphorus trichloride
CCl4 Carbon tetrachloride
N2O5 Dinitrogen pentoxide
CS2 Carbon disulfide
Practice!
SO2
N2O
NO2
CCl4
Cl2O7
PCl3
SF6
Si3N4
H2O
Sulfur Dioxide
Dinitrogen Monoxide
Nitrogen Dioxide
Carbon Tetrachloride
Dichlorine Heptaoxide
Phosphorus Trichloride
Sulfur Hexaflouride
Trisilicon Tetranitride
Water - can use
common name
Flow Chart for Naming Binary Compounds