measure specifications
advertisement

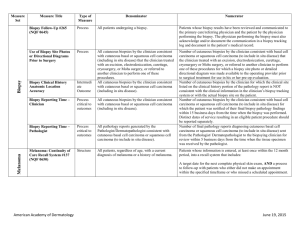
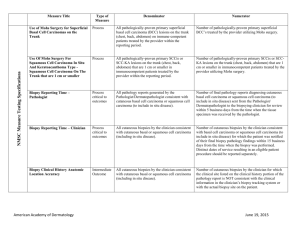
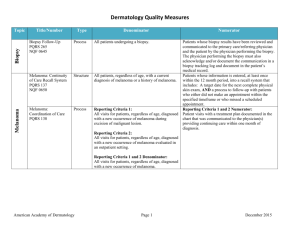
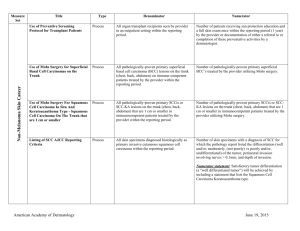
Title/Number Type Denominator Numerator Biopsy Follow-Up PQRS 265/NQF 0645 Process All patients undergoing a biopsy. Patients whose biopsy results have been reviewed and communicated to the primary care/referring physician and the patient by the physician performing the biopsy. The physician performing the biopsy must also acknowledge and/or document the communication in a biopsy tracking log and document in the patient’s medical record. Melanoma: Continuity of Care Recall System PQRS 137/NQF 0650 Structure All patients, regardless of age, with a current diagnosis of melanoma or a history of melanoma. Patients whose information is entered, at least once within the 12 month period, into a recall system that includes: Biopsy Measure Set Melanoma A target date for the next complete physical skin exam, AND a process to follow-up with patients who either did not make an appointment within the specified timeframe or who missed a scheduled appointment. Melanoma: Coordination of Care PQRS 138 Process Reporting Criteria 1: All visits for patients, regardless of age, diagnosed with a new occurrence of melanoma during excision of malignant lesion. Reporting Criteria 1 and 2 Numerator: Patient visits with a treatment plan documented in the chart that was communicated to the physician(s) providing continuing care within one month of diagnosis. Reporting Criteria 2: All visits for patients, regardless of age, diagnosed with a new occurrence of melanoma evaluated in an outpatient setting. Reporting Criteria 1 and 2 Denominator: All visits for patients, regardless of age, diagnosed with a new occurrence of melanoma. American Academy of Dermatology June 19, 2015 Measure Set Title/Number Melanoma: Overutilization of Imaging Studies in Melanoma PQRS 224/NQF 0562 Type Process Denominator Reporting Criteria 1: Patients with a current diagnosis of Stage 0 through IIC melanoma without signs or symptoms suggesting systemic spread. Numerator Reporting Criteria 1 and 2 Numerator: Patients for whom no diagnostic imaging studies were ordered. Reporting Criteria 1 Denominator: All patients, regardless of age, with a current diagnosis of Stage 0 through IIC melanoma, without signs or symptoms suggesting systematic spread, seen for an office visit during the one-year measurement period. Reporting Criteria 2: Patients with a history of any stage melanoma without signs or symptoms suggesting systemic spread. Non-Melanoma Skin Cancer Reporting Criteria 2 Denominator: All patients, regardless of age, with a history of melanoma of any stage, without signs or symptoms suggesting systemic spread, seen for an office visit during the one-year measurement period. Use of Preventive Screening Protocol for Transplant Patients Process All organ transplant recipients seen by provider in an outpatient setting within the reporting period. Number of patients receiving sun protection education and a full skin exam once within the reporting period (1 year) by the provider or documentation of either a referral to or completion of these preventative activities by a dermatologist. Use of Mohs Surgery for Superficial Basal Cell Carcinomas on the Trunk Process All pathologically-proven primary superficial basal cell carcinoma (BCC) lesions on the trunk (chest, back, abdomen) on immune-competent patients treated by the provider within the reporting period. Number of pathologically-proven primary superficial BCC’s treated by the provider utilizing Mohs surgery. American Academy of Dermatology June 19, 2015 Measure Set Title/Number Type Denominator Numerator Use Of Mohs Surgery For Squamous Cell Carcinoma In Situ And Keratoacanthoma Type Squamous Cell Carcinoma On The Trunk that are 1 cm or smaller Process All pathologically-proven primary SCCis or SCCKA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider within the reporting period. Number of pathologically-proven primary SCCis or SCC-KA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider utilizing Mohs surgery. Listing of SCC AJCC Reporting Criteria Process All skin specimens diagnosed histologically as primary invasive cutaneous squamous cell carcinoma within the reporting period. Number of skin specimens with a diagnosis of SCC for which the pathology report listed the differentiation (well and/or, moderately, (not poorly) vs poorly and/or, undifferentiated) of the tumor, perineural invasion involving nerves > 0.1mm, and depth of invasion. Numerator statement: Satisfactory tumor differentiation (a “well differentiated tumor”) will be achieved by including a statement that lists the Squamous Cell Carcinoma Keratoacanthoma type. Listing of BCC Subtyping on the Biopsy Report Process All skin specimens diagnosed histologically as cutaneous basal cell carcinoma within the reporting period. Number of skin specimens with a diagnosis of BCC for which the pathology report listed the histopathological BCC subtype. Documentation of Patient Input for Treatment Type Process All patients treated for at least one superficial basal cell carcinoma or squamous cell carcinoma in situ by scalpel-based excisional surgery (including standard excision and Mohs surgery) within the reporting period. Number of patients for whom there is documentation of patient (or legal caregiver) input regarding treatment options at least once per reporting period. Documentation of discussion w/patients about potential use of fielddirected therapies prior to destruction of Actinic Keratoses (17004 code) Process All patients with destruction of 15 or more AKs in a single visit (code 17004). Number of patients with destruction of 15 or more AKs who have documentation of a discussion of risks and benefits of using field-directed therapy with their physician. American Academy of Dermatology Numerator Instructions: This measure will be reported at least once per 12 month reporting period. To satisfy this measure, patients undergoing destruction of 15 or more AKs need a statement such as the following documented in their chart at least once during the reporting period: “The patient was informed of the risks and benefits of using field-directed therapy and their questions were answered.” June 19, 2015 Measure Set Title/Number Type Denominator Numerator Use of Biopsy Site Photos or Directional Diagrams Prior to Surgery Process All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease) that the clinician treated with an excision, electrodesiccation, curettage, cryosurgery, or Mohs surgery, or referred to another clinician to perform one of these procedures. Number of cutaneous biopsies by the clinician consistent with basal cell carcinoma or squamous cell carcinoma (to include in situ disease) that the clinician treated with an excision, electrodesiccation, curettage, cryosurgery or Mohs surgery, or referred to another clinician to perform one of these procedures for which a biopsy site photo or detailed directional diagram was made available to the operating provider prior to surgical treatment for use in his or her pre-op evaluation. Biopsy Clinical History Anatomic Location Accuracy Intermediate Outcome All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician for which the clinical site listed on the clinical history portion of the pathology report is NOT consistent with the clinical information in the clinician’s biopsy tracking system or with the actual biopsy site on the patient. Biopsy Reporting Time – Clinician Process critical to outcomes All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician consistent with basal cell carcinoma or squamous cell carcinoma (to include in situ disease) for which the patient was notified of their final biopsy pathology findings within 15 business days from the time when the biopsy was performed. Distinct dates of service resulting in an eligible patient procedure should be reported separately. Biopsy Reporting Time – Pathologist Process critical to outcomes All pathology reports generated by the Pathologist/Dermatopathologist consistent with cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease). Number of final pathology reports diagnosing cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease) sent from the Pathologist/ Dermatopathologist to the biopsying clinician for review within 5 business days from the time when the tissue specimen was received by the pathologist. American Academy of Dermatology June 19, 2015 Measure Set Title/Number Assessment of Psoriasis Disease Activity Type Process Denominator All patients with a diagnosis of plaque psoriasis. Numerator Patients with disease activity assessed by a standardized descriptive or numeric scale or composite index.* Psoriasis *Assessment and classification of disease activity: Scales and instruments are examples and cut-off points can differ by scale. Standardized descriptive or numeric scales and/or composite indexes could include but are not limited to: Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), Physician’s Global Assessment (PGA), Dermatology Life Quality Index (DLQI). Numerator Instructions: To satisfy this measure, psoriasis activity should be measured using any of the above or other scales or instruments at least once during the reporting period for each patient with a diagnosis of plaque psoriasis who presents for an office visit. Assessment for Psoriatic Arthritis MUC 136/ MAPX3274 Process All patients with a diagnosis of psoriasis. Patients who are “screened”* for psoriatic arthritis. *“Screening” for psoriatic arthritis must, at a minimum, include inquiry about the presence or absence of joint symptoms including any of the following: morning stiffness, pain, redness, and/or swelling of joints. If a dermatologist wishes to perform additional optional screening measures, these may include physical examination (e.g. visualization of joints, surrounding structures (entheses) and fingers/toes for dactylitis) and/or use of a validated psoriatic arthritis screening instrument (Psoriatic Arthritis Screening and Evaluation), ToPAS (Toronto Psoriatic Arthritis Screening) or PEST (Psoriasis Epidemiology Screening Tool). Numerator Instructions: To satisfy this measure, presence or absence of joint symptoms should be documented at least once during the reporting period. American Academy of Dermatology June 19, 2015 Measure Set Title/Number Type Tuberculosis Prevention for Psoriasis, Psoriatic Arthritis and Rheumatoid Arthritis Patients on a Biological Immune Response Modifier PQRS 337 Process Clinical Response to Oral Systemic or Biologic Medications MUC 134/MAP X3726 Outcome Denominator All patients with a diagnosis of psoriasis, psoriatic arthritis, or rheumatoid arthritis who are on a biologic immune response modifier. Denominator Note: A patient would be considered denominator eligible for Measure #337 for reporting purposes, if the patient meets the denominator criteria with diagnosis of psoriasis or psoriatic arthritis or rheumatoid arthritis AND is on a biologic immune response modifier prescribed by the provider being evaluated for the measure. All patients with a diagnosis of psoriasis and treated with an oral systemic or biologic medication for psoriasis for at least 6 months. Numerator Patients who have a documented negative annual TB screening or have documentation of the management of a positive TB screening test with no evidence of active tuberculosis, confirmed through use of radiographic imaging (i.e. chest x-ray, CT). Patients who have a documented physician global assessment (PGA; 6-point scale), body surface area (BSA), psoriasis area and severity index (PASI) and/or dermatology life quality index (DLQI) that meet any one of the below specified benchmarks. Numerator Instructions: To satisfy this measure, a patient must achieve any ONE of the following: a. PGA (6-point scale) ˂ 2 (clear to mild skin disease) b. BSA < 3% (mild disease) c. PASI < 3 (no or minimal disease) d. DLQI < 5 (no effect or small effect on patient’s quality of life). American Academy of Dermatology June 19, 2015