Measure Set Title Type Denominator Numerator Non
advertisement

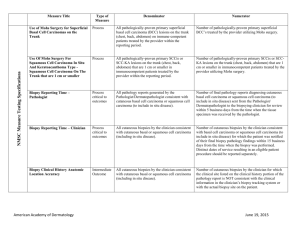
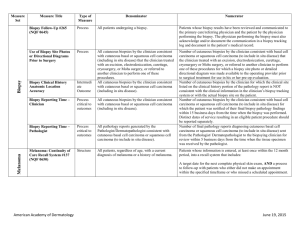
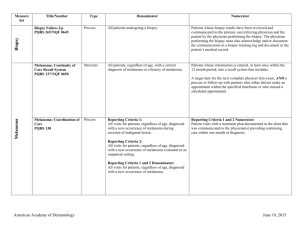
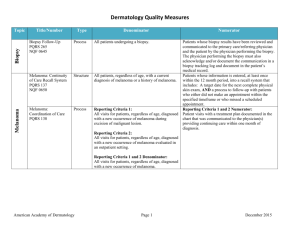
Non-Melanoma Skin Cancer Measure Set Title Type Denominator Numerator Use of Preventive Screening Protocol for Transplant Patients Process All organ transplant recipients seen by provider in an outpatient setting within the reporting period. Number of patients receiving sun protection education and a full skin exam once within the reporting period (1 year) by the provider or documentation of either a referral to or completion of these preventative activities by a dermatologist. Use of Mohs Surgery for Superficial Basal Cell Carcinomas on the Trunk Process All pathologically-proven primary superficial basal cell carcinoma (BCC) lesions on the trunk (chest, back, abdomen) on immune-competent patients treated by the provider within the reporting period. Number of pathologically-proven primary superficial BCC’s treated by the provider utilizing Mohs surgery. Use of Mohs Surgery For Squamous Cell Carcinoma In Situ And Keratoacanthoma Type - Squamous Cell Carcinoma On The Trunk that are 1 cm or smaller Process All pathologically-proven primary SCCis or SCC-KA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider within the reporting period. Number of pathologically-proven primary SCCis or SCCKA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider utilizing Mohs surgery. Listing of SCC AJCC Reporting Criteria Process All skin specimens diagnosed histologically as primary invasive cutaneous squamous cell carcinoma within the reporting period. Number of skin specimens with a diagnosis of SCC for which the pathology report listed the differentiation (well and/or, moderately, (not poorly) vs poorly and/or, undifferentiated) of the tumor, perineural invasion involving nerves > 0.1mm, and depth of invasion. Numerator statement: Satisfactory tumor differentiation (a “well differentiated tumor”) will be achieved by including a statement that lists the Squamous Cell Carcinoma Keratoacanthoma type. American Academy of Dermatology June 19, 2015 Listing of BCC Subtyping on the Biopsy Report Process All skin specimens diagnosed histologically as cutaneous basal cell carcinoma within the reporting period. Number of skin specimens with a diagnosis of BCC for which the pathology report listed the histopathological BCC subtype. Documentation of Patient Input for Treatment Type Process All patients treated for at least one superficial basal cell carcinoma or squamous cell carcinoma in situ by scalpel-based excisional surgery (including standard excision and Mohs surgery) within the reporting period. Number of patients for whom there is documentation of patient (or legal caregiver) input regarding treatment options at least once per reporting period. Documentation of discussion w/patients about potential use of field-directed therapies prior to destruction of Actinic Keratoses (17004 code) Process All patients with destruction of 15 or more AKs in a single visit (code 17004). Number of patients with destruction of 15 or more AKs who have documentation of a discussion of risks and benefits of using field-directed therapy with their physician. Numerator Instructions: This measure will be reported at least once per 12 month reporting period. To satisfy this measure, patients undergoing destruction of 15 or more AKs need a statement such as the following documented in their chart at least once during the reporting period: “The patient was informed of the risks and benefits of using field-directed therapy and their questions were answered.” American Academy of Dermatology June 19, 2015 Use of Biopsy Site Photos or Directional Diagrams Prior to Surgery (NMSC) Process All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease) that the clinician treated with an excision, electrodesiccation, curettage, cryosurgery, or Mohs surgery, or referred to another clinician to perform one of these procedures. Number of cutaneous biopsies by the clinician consistent with basal cell carcinoma or squamous cell carcinoma (to include in situ disease) that the clinician treated with an excision, electrodesiccation, curettage, cryosurgery or Mohs surgery, or referred to another clinician to perform one of these procedures for which a biopsy site photo or detailed directional diagram was made available to the operating provider prior to surgical treatment for use in his or her pre-op evaluation. Biopsy Clinical History Anatomic Location Accuracy (NMSC) Intermediate Outcome All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician for which the clinical site listed on the clinical history portion of the pathology report is NOT consistent with the clinical information in the clinician’s biopsy tracking system or with the actual biopsy site on the patient. Biopsy Reporting Time (NMSC)– Clinician Process critical to outcomes All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician consistent with basal cell carcinoma or squamous cell carcinoma (to include in situ disease) for which the patient was notified of their final biopsy pathology findings within 15 business days from the time when the biopsy was performed. Distinct dates of service resulting in an eligible patient procedure should be reported separately. Biopsy Reporting Time (NMSC) – Pathologist Process critical to outcomes All pathology reports generated by the Pathologist/Dermatopathologist consistent with cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease). Number of final pathology reports diagnosing cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease) sent from the Pathologist/ Dermatopathologist to the biopsying clinician for review within 5 business days from the time when the tissue specimen was received by the pathologist. American Academy of Dermatology June 19, 2015