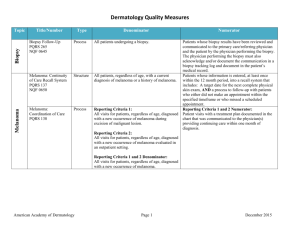
Measure Title Type of Measure Denominator Numerator NMSC
advertisement
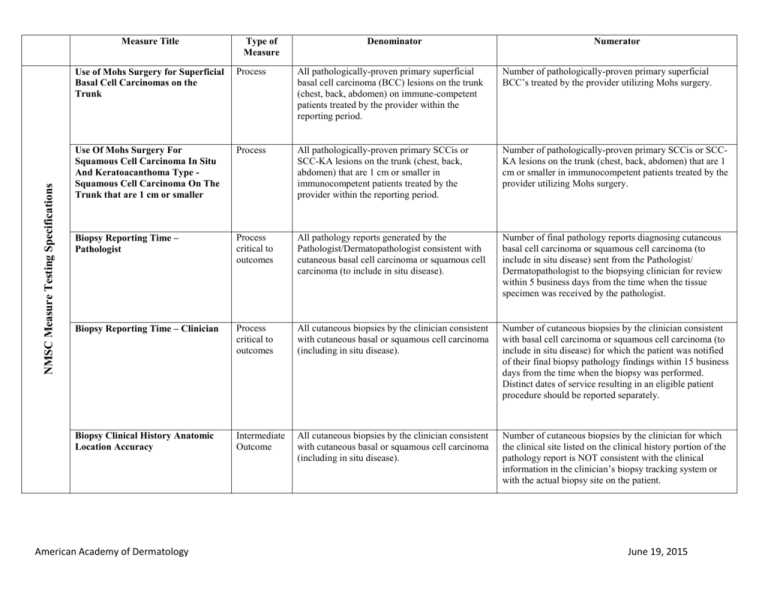
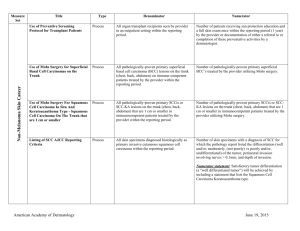
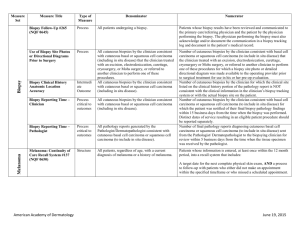
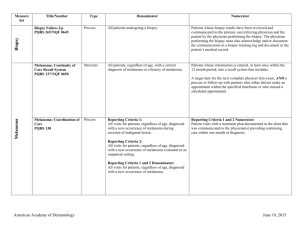
NMSC Measure Testing Specifications Measure Title Type of Measure Denominator Numerator Use of Mohs Surgery for Superficial Basal Cell Carcinomas on the Trunk Process All pathologically-proven primary superficial basal cell carcinoma (BCC) lesions on the trunk (chest, back, abdomen) on immune-competent patients treated by the provider within the reporting period. Number of pathologically-proven primary superficial BCC’s treated by the provider utilizing Mohs surgery. Use Of Mohs Surgery For Squamous Cell Carcinoma In Situ And Keratoacanthoma Type Squamous Cell Carcinoma On The Trunk that are 1 cm or smaller Process All pathologically-proven primary SCCis or SCC-KA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider within the reporting period. Number of pathologically-proven primary SCCis or SCCKA lesions on the trunk (chest, back, abdomen) that are 1 cm or smaller in immunocompetent patients treated by the provider utilizing Mohs surgery. Biopsy Reporting Time – Pathologist Process critical to outcomes All pathology reports generated by the Pathologist/Dermatopathologist consistent with cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease). Number of final pathology reports diagnosing cutaneous basal cell carcinoma or squamous cell carcinoma (to include in situ disease) sent from the Pathologist/ Dermatopathologist to the biopsying clinician for review within 5 business days from the time when the tissue specimen was received by the pathologist. Biopsy Reporting Time – Clinician Process critical to outcomes All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician consistent with basal cell carcinoma or squamous cell carcinoma (to include in situ disease) for which the patient was notified of their final biopsy pathology findings within 15 business days from the time when the biopsy was performed. Distinct dates of service resulting in an eligible patient procedure should be reported separately. Biopsy Clinical History Anatomic Location Accuracy Intermediate Outcome All cutaneous biopsies by the clinician consistent with cutaneous basal or squamous cell carcinoma (including in situ disease). Number of cutaneous biopsies by the clinician for which the clinical site listed on the clinical history portion of the pathology report is NOT consistent with the clinical information in the clinician’s biopsy tracking system or with the actual biopsy site on the patient. American Academy of Dermatology June 19, 2015