Path Chapter 18 p855-881 [4-20
advertisement

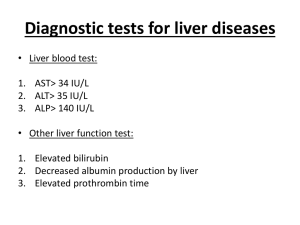
Path Chapter 18: Liver and Biliary Tract (pages 855-881) Autoimmune hepatitis – chronic and progressive hepatitis from T cell autoimmunity - - - Liver injury comes from CD4+ and CD8+ T cells making interferon-γ, and from TC cell attacks It may be caused by a defect in regulatory T cells, allowing uncontrolled activation of selfreactive lymphocytes Autoimmune hepatitis often happens with other autoimmune disorders (ex: celiac’s) The trigger for autoimmune hepatitis can be a virus, drug, or herb like black cohosh Most cases of autoimmune hepatitis are in women, and whites are most affected Autoimmune hepatitis shows no serum markers of viral infection, but high serum IgG and γglobulin, and high serum autoantibodies There are two types of autoimmune hepatitis: o Type 1 auto immune hepatitis – characterized by presence of antibodies antinuclear (ANA), anti-smooth muscle (SMA), anti-actin (AAA), and anti-soluble liver antigen/liverpancreas antigen (anti-SLA/LP) More common o Type 2 AI hepatitis – characterized by presence of anti-liver kidney microsome-1 (ALKM1) antibodies, which mostly target anti-liver cytosol-1 (ACL-1) and CYP2D6 Autoimmune hepatitis is mainly marked by inflammatory infiltrate of lymphocytes and plasma cells o A characteristic of autoimmune hepatitis is clusters of plasma cells Symptomatic autoimmune hepatitis will cause lots of destruction and scarring of the liver Can lead to fulminant hepatitis and hepatic encephalopathy within 2 months About half of autoimmune hepatitis leads to death in 6 months, and half of survivors develop cirrhosis Treat with prednisone, with maybe azathioprine as well After liver transplant, a lot of them have autoimmune hepatitis return About 10% of adverse drug rxns are drug-caused liver injury, and it’s the most common cause of fulminant hepatitis in the US Ways drugs cause injury to the liver: - Direct injury – the drug is toxic to hepatocytes or bile epithelial cells The liver converts the drug into a toxin The drug converts a cell protein into an antigen the immune system reacts against Drug rxns can either be predictable (intrinsic) or unpredictable (idiosyncratic) - Predictable drug rxns – can happen in anyone who receives enough of the drug to cause the rxn Unpredictable drug rxns – depends on the host and not the drug dosage, especially the rate the host metabolizes the agent, and the intensity of the immune response o Should be suspected in anyone taking a drug who develops liver damage - - - o Unpredictable rxns are characterized by high bilirubin Usually adults are affected more than kids, and women more than men Chlorpromazine –causes cholestasis(bile backs up into liver) in patients who metabolize it slowly Halothane – anesthetic that can cause fatal hepatitis Drug-induced chronic hepatitis acts the exact same as viral hepatitis, so serum markers of viral infection are critical in telling the difference Page 856 – table of drugs and what they do to the liver o Acetaminophen, carbon tetrachloride, and alcohol cause predictable drug rxns in the liver o Sulfonamides, allopurinol, and α-methyldopa, cause unpredictable drug rxns Acetaminophen is the leading cause of drug-induced acute liver failure The most common cause of unpredictable rxns are antibiotics, nonsteroidal analgesics, and antiseizure meds Reye syndrome – rare syndrome characterized by accumulation of fat droplets in hepatocytes, called microvesicular steatosis, due to mitochondria dysfunction o Also shows brain problems, and can be fatal o Reye’s is thought to be caused by aspirin (acetylsalicylic acid) This is why you should avoid giving children aspirin for a fever Long term use of methotrexate for psoriasis, can cause liver injury, including hepatic steatosis and fibrosis Drug-caused liver disease usually goes away when you remove the drug Exposure to a toxin or drug should always be included as a differential diagnosis in liver disease Alcoholic liver disease – excessive alcohol (ethanol) consumption is the leading cause of liver disease in most western countries - - 3 forms of alcoholic liver disease – they overlap a lot o Pics of each form – page 857 o Hepatic steatosis (fatty liver disease) o Alcoholic hepatitis o Cirrhosis Hepatic steatosis (fatty liver) – shows fatty change and perivenular fibrosis o Even after moderate intake of alcohol, microvesicular lipid droplets accumulate in hepatocytes o Chronic intake leads to big, clear macrovesicular globules of lipid, that compress and displace the hepatocyte nucleus to the periphery of the cell o The fatty liver is large, soft, yellow, and greasy o Fibrosis will take place the longer you keep drinking after fatty liver starts o Fatty change to the liver is completely reversible if you quit drinking alcohol o Hepatic steatosis can manifest as enlarged liver, with mild elevation of serum bilirubin and alkaline phosphatase o - - - - Fatty liver usually doesn’t severely impair the liver, and you can treat by not drinking alcohol and having a good diet Alcoholic hepatitis-characterized by swelling & necrosis, Mallory bodies,neutrophil rxn,& fibrosis o Hepatocyte swelling (aka ballooning) and necrosis The swelling is from accumulation of fat, water, and proteins that would normally be exported out of the hepatocyte o Mallory bodies - eosinophilic cytoplasmic clumps in hepatocytes They’re characteristic of, but not specific for, alcoholic liver disease The clumps are made of cytokeratin and proteins like ubiquitin o Neutrophilic rxn – neutrophils enter the liver and accumulate around dying hepatocytes o Fibrosis – alcoholic hepatitis almost always activates stellate cells and fibroblasts in the liver, causing fibrosis Most often, fibrosis is in the sinusoids or around a vein (perivenular) o Alcoholic hepatitis usually shows up acutely after an episode of heavy drinking o Can have anywhere from minimal to fulminant hepatic failure o Symptoms of alcoholic hepatitis include malaise, anorexia, weight loss, upper abdominal discomfort, tender enlarged liver o Labs for alcoholic hepatitis will show hyperbilirubinemia, increased alkaline phosphatase, and neutrophil leukocytosis (high #’s of neutrophils) o Each bout of alcoholic hepatitis has a 10-20% risk of death o Within a few years, 1/3 of cases of alcoholic hepatitis develop cirrhosis o Sometimes stopping alcohol and good diet works, but sometimes it doesn’t and the hepatitis progresses to cirrhosis Cirrhosis – the final and irreversible form of alcoholic liver disease o At first, a cirrhotic liver is yellow-tan, fatty, and enlarged o Over years, it transforms into a brown, shrunken, nonfatty organ o The portal tracts and sinusoids will show fibrosis o Nodules form by normal liver in between the fibrosis o Time brings more nodules, giving the liver a “hobnail” appearance on it’s surface o Ischemic necrosis and destruction by fibrosis eventually creates big areas of tough pale scar tissue, called Laenne cirrhosis o Lab findings in alcoholic cirrhosis show elevated serum aminotransferase and alkaline phoshpatase, hyperbilirubinemia, hypoproteinemia (low clotting factors, albumin, and globulins), and anemia Short term ingestion of up to 6 beers over 1-several days, will cause a mild, reversible hepatic steatosis (fatty liver) o Drinking any more than that over that period causes significant risk for severe liver injury o Daily ingestion of double that for 10-20 years will cause severe liver injury Only 10-15% of alcoholics will develop cirrhosis o This means other factors must also influence the development and severity of alcoholic liver disease o - - - - These factors include – gender, race, and genetics Gender – women are more susceptible Despite this, most cases are men Estrogen increases gut permeability to endotoxins, which increases expression of the LPS receptor CD14 in Kupffer cells, which causes increased making of pro-inflammatory cytokines Race – blacks have more cirrhosis than whites, even when they drink the same amount Genetics – detoxing enzymes and cytokine promoters can be messed up ALDH*2 – name for mutation to aldehyde-dehydrogenase (ALDH) o This mutation is found in half of Asians, and makes ALDH have low activity o People who are homozygous for ALDH*2 can’t oxidize acetaldehyde, so they can’t tolerate alcohol Iron overload and viral hepatitis will increase severity of alcoholic liver disease Alcohol causes steatosis, dysfunction of mitochondria and cell membranes, hypoxia, and oxidative stress Hepatocellular steatosis (fatty liver) results from: o Shunting of normal stuff away from catabolism and towards lipid making, due to making of excess reduced NADH (nicotanamide adenine dinucleotide) by alcohol dehydrogenase and acetaldehyde dehydrogenase, the two main enzymes for alcohol metabolism o Problems assembling and secreting lipoproteins o Increased peripheral catabolism of fat Acetaldehyde is the major intermediate metabolite of alcohol o Acetaldehyde induces lipid peroxidation, and acetaldehyde-protein adduct making Both further disrupt the cytoskeleton and membrane of the hepatocyte Cytochrome P-450 metabolism produces reactive oxygen species (ROS) that react with cell proteins, damage membranes, and change hepatocyte function Alcohol caused problems with liver metabolism of methionine causes decreased glutathione levels in the hepatocyte, making it more prone to oxidative injury Alcohol triggers enzymes to break it down, and those same enzymes are now working and enhance conversion of other drugs, like acetaminophen, to toxic metabolites Alcohol can take up more calories than it should in an alcoholic diet, causing malnutrition from lack of vitamins and other nutrients o This is made worse by impaired digestive function, from chronic GI mucosa damage, and pancreatitis Alcohol causes the release of bacterial endotoxin from the gut, into the portal circulation o This triggers inflammatory responses in the liver - - - Alcohol also triggers release of endothelins from sinusoid endothelial cells, causing vasoconstriction, and contraction of myofibroblasts (activated stellate cells), leading to a decrease in liver sinusoid perfusion o This can lead to portal hypertension To summarize, alcoholic liver disease is a chronic disorder featuring steatosis, hepatitis, progressive fibrosis, cirrhosis, and problems with vascular perfusion o It can be thought of as a slightly harmful effect on hepatocytes at first, that causes them to respond in a more and more pathogenic manner each time it sees stimuli The main causes of death from alcoholic liver disease are hepatic coma, massive GI bleed, infection (they’re at risk for them), hepatorenal syndrome after an episode of alcoholic hepatitis, and hepatocellular carcinoma The most common acquired metabolic disorder of the liver is non-alcoholic fatty liver disease (NAFLD) - - - - NAFLD – group of conditions that have in common that there’s hepatic steatosis (fatty liver), and that they don’t drink alcohol enough to warrant fatty liver NAFLD is the most common cause of chronic liver disease in the US, affecting 1/3 of americans NAFLD includes: o Hepatic steatosis (fatty liver) o Steatosis accompanied by minor, nonspecific inflammation o Non-alcoholic steatohepatitis (NASH) Steatosis (fatty liver) with or w/o inflammation, is usually stable without any clinical problems NASH has hepatocyte injury that can progress to cirrhosis in up to 1/5 of cases o NASH shows hepatocyte ballooning, lobular inflammation, and steatosis o As NASH progresses, fibrosis happens o NASH is strongly associated to metabolic syndrome – like obesity, diabetes, and ↑lipids It’s thought more than 2/3 of obese people have some form of NAFLD In NAFLD, lipids accumulate in the liver, and oxidative stress acts upon these lipids, causing lipid peroxidation and the release of lipid peroxides, which can make reactive oxygen species Simple steatosis is usually asymptomatic Liver biopsy is the best way to diagnose NASH Labs for NASH shows elevated serum AST and ALT o The AST/ALT ratio is usually less than 1, as opposed to alcoholic fatty liver, where the ratio is usually 2 or above If NASH shows symptoms, it will show fatigue and right side abdominal discomfort caused by hepatomegaly Cardiovascular disease is the most common cause of death in NASH Steatosis morphology shows large (macrovesicular) and small (microvesicular) droplets of fat in hepatocytes o The most benign form of steatosis shows no liver inflammation, cell death, or scarring, despite high serum liver enzymes (AST and ALT) - NASH (steatohepatitis) is characterized by steatosis and diffuse inflammation with mainly neutrophils, Mallory bodies, hepatocyte death (by ballooning degeneration and apoptosis), and sinusoid fibrosis Hemochromatosis – homozygous recessive disorder of excessive accumulation of iron, most of which is deposited in the liver and pancreas - - - - Other places iron accumulates is the heart, joints, or endocrine organs The excessive iron comes from excessive iron absorption from the intestines Aka primary hemochromatosis Hemosiderosis (aka secondary hemochromatosis) – acquired accumulation of iron in the tissues, like in transfusions A lot of the body’s iron is normally stored in the liver, almost all of which is in the hepatocytes Hemochromatosis is characterized by: o Necrosis with nodules o Diabetes – in most cases o Skin pigmentation – in most cases o The iron accumulation happens for life, but it doesn’t cause injury for a while cause it’s slow and progressive – notice it in 50’s-60’s o More common in males – since women lose iron through menstruation to delay the process There is no regulation to iron excretion, so the body closely watches how much it needs to absorb to keep up o In hemochromatosis, regulation of dietary iron absorption in the intestine is abnormal, leading to iron accumulation, mainly in the liver Excessive iron is directly toxic to tissues: o Iron catalyzes free radical rxns, causing lipid peroxidation o Iron stimulates fibrosis, by activating hepatic stellate cells o Iron and free radicals it makes, can interact with DNA, causing lethal cell injury or increase the chances of hepatocellular carcinoma Iron damage is reversible if the cell isn’t fatally injured The main regulator of iron absorption is the protein hepcidin o Hepcidin is made from the HAMP gene in hepatocytes, and also works as an antibiotic o Inflammatory cytokines and iron both increase transcription of hepcidin o Iron deficiency, hypoxia, and poor erythropoiesis all decrease making of hepcidin o Hepcidin binds to the iron transport channel ferroportin (FPN) and internalizes it This prevents release of iron from intestine cells and macrophage, so the job of hepcidin is to lower plasma iron levels o Problems with hepcidin cause iron overload o Proteins that regulate iron metabolism by regulating hepcidin include hemojuvelin (HJV), transferrin receptor 2 (TfR2), and HFE o - - - - Mutations to hepcidin, hemojuvelin, TfR2, and HFE cause hemochromatosis by a lack of hepcidin expression HFE mutations are most common Mutations of HAMP and hemojuvelin cause a severe form called juvenile hemochromatosis Mutations of HFE and transferrin receptor 2 cause classic hereditary adult hemochromatosis, but it’s almost always HFE HFE is close to the HLA gene, and codes for HLA class 1- like molecules that regulate intestine absorption of dietary iron The most common HFE mutation is called C282Y, and is seen in most cases of hereditary hemochromatosis, and more common in whites o It’s so common it’s makes hereditary hemochromatosis one of the most common genetic disorders in humans o But despite how common it is, it usually doesn’t lead to clinical disease The characteristic morphologic changes in hemochromatosis are deposition of hemosiderin in the liver, pancreas, heart, etc.; cirrhosis, and pancreatic fibrosis o The iron looks like gold-yellow granules in the cytoplasm of hepatocytes, which will stain blue with Prussian blue stain o The liver then enlarges, & fibrosis with nodules leads to cirrhosis in a very pigmented liver o The pancreas will also become pigmented and have fibrosis o The heart is often enlarged and has hemosiderin granules, causing an obvious brown color to the myocardium o The skin becomes pigmented a little by hemosiderin deposited into skin macrophage and fibroblasts, and a lot from increased melanin making This combo makes the skin look “slate gray” o Hemosiderin in the joints can cause tenosynovitis, and calcium pyrophosphate can come along too to get the cartilage, causing a disabling polyarthritis called pseudo-gout o The testes may atrophy – possible due to pituitary iron storage, decreasing FSH and LH Classic hemochromatosis is more common in males, and rarely seen before age 40 The main symptoms of hemochromatosis are enlarged liver, abdominal pain, skin pigmentation (especially in sunexposed areas), problems with glucose metabolism or diabetes from destruction of islet cells, heart problems, and arthritis The classic triad of pigment cirrhosis with enlarged liver, skin pigmentation, and diabetes mellitus, might not show up until late in the disease Death from hemochromatosis can be from heart problems, cirrhosis, and hepatocellular cancer o Hemochromatosis increases the risk for hepatocellular cancer, and treating the overload won’t decrease the risk again Lab tests will show very high serum iron and ferritin Heterozygotes also accumulate iron, but not enough that it causes much tissue damage - The most common causes of hemosiderosis are disorders of ineffective erythropoiesis (like severe thalassemia), and myelodysplastic syndromes Wilson disease – autosomal recessive disorder that causes impaired copper excretion into bile and copper incorporation into ceruloplasmin, causing toxic accumulation of copper in tissues - - - - - Caused by mutation to the ATP7B gene The copper buildup is mainly in the liver, brain, and eye Normally, about half of ingested copper is absorbed in the small intestine o It then complexes with albumin and histidine, and goes through the portal system, where it lets go of the carriers and is taken up by hepatocytes Copper is incorporated into enzymes, and also binds to apoceruplasmin, forming ceruplasmin, which is released into blood Excess copper goes into the bile Ceruplasmin holds close to all of the plasma copper Circulating ceruplasmin is eventually endocytosed into the liver and broken down in lysosomes, and the copper is released into the blood The ATP7B gene codes a copper transport protein on hepatocytes o Most cases of Wilson disease have heterozygotes, with 2 mutations, but each mutation to each allele is a different kind of mutation (called a compound heterozygote) o Deficiency in ATP7B causes a decrease in copper transport into bile, impairs making of ceruplasmin, and inhibits ceruplasmin release into blood o This causes copper to accumulate in the liver, and a decrease in circulating ceruplasmin Too much copper in the liver is toxic to it, because of making of reactive oxygen species Once the liver maxes out on how much copper it can convert to ceruplasmin, free copper spills into the blood, causing hemolysis and problems in the brain, eye, etc., causing symptoms The liver damage from Wilson’s disease shows fatty change (steatosis) and acute hepatitis o When it gets to chronic hepatitis, you see inflammation and hepatocyte necrosis, and macrovesicular steatosis Also see vacuoles in the hepatocyte nuclei and Mallory bodies o The chronic hepatitis can progress to cirrhosis Need to show excess copper in the liver to diagnose, since Wilson’s disease can look like other liver diseases In the brain, the copper targets the basal ganglia, causing atrophy Nearly all people with Wilson’s disease will develop eye lesions called Keyser-Fleischer rings o It looks like green-brown copper deposits in the limbus (outer edge of the eye) o REMEMBER SCRUBS FOR WILSONS DISEASE – “It looks like a little golden ring” The most common presentation of Wilson disease shows chronic liver disease o Neuro symptoms are also common and include behavior changes, psychosis, or a Parkinson-like tremor - You diagnose Wilson disease based on a decrease in serum ceruloplasmin, increased liver copper (most sensitive and accurate test), & ↑ urinary excretion of copper (most specific) Serum copper won’t help, since levels fluctuate throughout the disease α1-antitrypsin deficiency – autosomal recessive disorder - - - α1-antitrypsin’s job is to inhibit proteases, especially neutrophil elastase, cathepsin G, and proteinase 3, which are all normally released from neutrophils at sites of inflammation α1-antitrypsin deficiency leads to pulmonary emphysema, because the activity of destructive proteases is not inhibited Protein also accumulates in the liver, causing liver disease α1-antitrypsin is mainly made by hepatocytes, and is a serine protease inhibitor (serpin) The gene for α1-antitrypsin makes many versions of it o To name them, you use “Pi” to mean protease inhibitor, and then give an alphabetic letter to it for how far it migrated on electrophoresis gel If you see 2 alphabetic letters, it’s telling you the genotype o The most common genotype is PiMM o The most common disease causing mutation is PiZ, and PiZZ homozygoes have only 1/10 the circulating α1-antitrypsin of normal o α1-antitrypsin is the most common genetic liver disorder in infants and children o Most mutated versions of α1-antitrypsin still have protein made and released o Mutations where protein aren’t released are because there’s something wrong with migration from the ER to the golgi Especially in PiZ The mutant protein is called α1AT-Z, and is abnormally folded and so it accumulates in the ER, causing ER stress and apoptosis But only up to 15% of these cases develop into liver disease α1-antitrypsin deficiency is characterized by round-oval eosinopihlic globular inclusions in hepatocyte cytoplasm o Usually the only indicators of liver disease are PAS-positive globules Up to 1/5 of newborns with α1-antitrypsin deficiency will have neonatal hepatitis with jaundice In adolescence, symptoms may be caused by hepatitis or cirrhosis Or α1-antitrypsin deficiency can remain silent until cirrhosis happens in mid-to-late life Neonatal cholestasis – prolonged conjugated hyperbilirubinemia in a neonate - Neonatal cholestasis is caused by cholangiopathies (especially biliary atresia), and neonatal hepatitis (term for anything causing conjugated hyperbilirubinemia in the neonate) Neonatal cholestasis should make you think there’s a toxic, metabolic, or infectious cause o However, half of cases have no known cause (idiopathic) o Common causes are biliary atresia and α1-antitrypsin deficiency - Need to differentiate whether the causes is obstructive or not, cause surgery for biliary atresia would make neonatal cholestasis worse if it has an unobstructive cause Symptoms of neonatal cholestasis are jaundice, dark urine, light or acholic (pale and clay colored) stools, and hepatomegaly Morphology of neonatal cholestasis is apoptosis and necrosis in the liver, giant cells replacing hepatocytes, cholestasis, mononuclear infiltrate, and hematopoiesis Diseases of the bile tract within in the liver – page 867 table 18-8 - - Secondary biliary cirrhosis – obstruction of the bile tree outside the liver cause damage to the bile tract in the liver o The first change seen is cholestasis (bile backs up into the liver) – reversible if you remove the obstruction o Secondary inflammation from bile obstruction causes fibrosis of the liver, which causes liver scarring and nodule making, causing secondary biliary cirrhosis o If there isn’t complete obstruction, an infection may get into the bile tree, called ascending cholangitis, making inflammation worse o The liver will be yellow-green in color, and other body tissues look icteric – page 867 pic o The liver is hard and looks granular o Fibrosis divides the liver into a “jigsaw” pattern o The smaller bile ducts proliferate o Cholestasis may show feathery degeneration (bile droplets) and bile lakes o Symptoms include itching, jaundice, malaise, dark urine, light stools, and enlarged liver Dark urine and light stools – signs of conjugated hyperbilirubinemia Itching is a symptom of high bilirubin o Lab findings show conjugated hyperbilirubinemia, and increased serum alkaline phosphatase, bile acids, and cholesterol Primary biliary cirrhosis (PBC) – inflammatory autoimmune disease that mainly affects the bile ducts in the liver o The main feature of PBC is inflammatory destruction of small bile ducts in the liver This is accompanied by portal inflammation, scarring, and progression to cirrhosis and liver failure o PBC mainly affects middle-aged women o Family members of people with PBC have an increased risk of getting it o Onset of PBC shows pruritus (itching) and fatigue, along with enlarged liver and eyelid xantehlasmas (cholesterol filled macrophage invade the nasal area) o Up to ½ of cases of PBC show hyperpigmentation due to melanin deposition, and inflammatory arthritis o After 20 years or so, cirrhosis develops, and you see portal hypertension with variceal bleeding, and hepatic encephalopathy o In PBC, serum alkaline phosphatase and cholesterol are almost always elevated Hyperbilirubinemia develops late and indicates the liver is failing o - - A characteristic of PBC used to diagnose, is antimitochondrial antibodies The antimitochondrial antibodies target pyrurate dehydrogenase Will also see elevated alkaline phosphatase & GGT, which indicate cholestasis o In the pre-cirrhosis stage of PBC, WBCs infiltrate the liver bile ducts and cause inflammation, which obstructs, causing secondary bile duct damage This leads to bile duct proliferation, necrosis, and inflammation upstream, and the liver develops cholestasis This relentless pattern of damage leads to cirrhosis o As PBC progresses, the liver turns green, and starts smooth, but finely ends up with small nodules and cirrhosis o The onset of PBC is insidious and may not show any symptoms for years When symptoms first show up, you get itching, fatigue, and abdominal discomfort Then secondary signs, like skin pigmentation, xanthelasmas, steatorrhea, and vitamin D malabsorption (from osteoporosis) show up End stage PBC shows jaundice, portal hypertension, and variceal bleeding o PBC increases your risk for hepatocellular carcinoma o The major cause of death from PBC is liver failure o PBC may also sow other autoimmune symptoms o Lab findings for PBC are the same as secondary biliary cirrhosis, plus elevated IgM autoantibodies Primary sclerosing cholangitis (PSC) – inflammation and fibrosis destroy bile ducts in and outside the liver, and remaining bile ducts become dilated o PSC shows characteristic “beading” of the bile ducts radiograph, and is caused by the dilations o PSC is often seen along wit inflammatory bowel disease, especially chronic ulcerative colitis o PSC happens in middle aged males mostly o PSC is a chronic cholestasis characterized by inflammation, fibrosis, and strictures of bile ducts o PSC is thought to be autoimmune, cause you see T cells in the ducts, lots of circulating autoantibodies, and it’s association with inflammatory bowel disease o First degree relatives of people with PSC have an increased risk o PSC can be asymptomatic, or show fatigue, itching, and jaundice o Severe PSC causes chornic liver disease, with weight loss, ascite, varices bleeding, and encephalopathy o PSC causes a risk for cholangiocarcinoma, pancreatitis, and hepatocellular carcinoma o Labs for PSC shows the same as secondary biliary cirrhosis(alkaline phosphatase, conjugated bilirubin, plasma cholesterol and bile acids), plus elevated serum IgM and hypergammaglobulinemia Page 867 – table 18-8 Von Meyenburg complexes-small clusters of dilated bile ducts near portal tracts o - - - - Von Meyenburg complexes are common, and aren’t a problem except as a possible differential diagnosis of metastases to the liver Polycystic liver disease – many diffuse cysts in the liver (pic page 870) Congenital hepatic fibrosis –shows many big abnormally shaped bile ducts, from persistence of the embryo bile duct, causing fibrosis their whole life o Portal tracts are enlarged by irregular collagen, which divides te liver into islands Caroli disease – large dilated bile ducts, and may have thickened bile o Caroli disease usually doesn’t happen alone, and goes along with portal tract fibrosis of congenital hepatic fibrosis o Has an increased risk for cholangiocarcinomas All 4 of these (von meyenburg complexes, polycystic liver disease, congenital hepatic fibrosis, and Caroli disease) can be associated with polycystic kidney disease o Liver cysts are the most common extrarenal manifestion of autosomal dominant polycystic kidney disease from mutation to PKD1, and happens in at least ¾ of patients o Congenital hepatic fibrosis is strongly associated with autosomal recessive (infant) PKD Alagille syndrome – rare disorder that affects many organs, but the liver part is there are no bile ducts in the portal tracts, caused by mutation to Jagged1-Notch system, used to help organs develop Problems with blood flow into the liver – will manifest as esophageal varices, splenomegaly, and/or intestinal congestion - Liver infarcts are rare, thanks to a double blood supply to the liver But a block in an intrahepatic branch of the hepatic artery can cause a local infarct Blockage of the extrahepatic portal vein can either not be a big deal, or be catastrophic o Usually causes abdominal pain, esophageal varices, and other issues involved with portal hypertension o Things that block the portal vein are infection, clotting disorders, trauma, pancreatitis, cancer, and cirrhosis, but most often the cause is unknown o Intrahepatic portal vein radicle can be obstructed by an acute thrombis, which won’t cause an ischemic infarct, be does cause red-blue discoloration, called an infarct of Zahn Problems with blood flow through the liver – will manifest as ascites, esophageal varices, enlarged liver, and elevated aminotransferases - - The most common intrahepatic cause of blood flow obstruction is cirrhosis In sickle cell, liver sinusoids get packed with sickled RBCs, causing liver necrosis Right sided heart failure causes congestion of the liver o The liver is enlarged, cyanotic, and has round edges o The centrilobular sinusoids will be congested Left sided heart failure or shock can cause cause liver hypoperfusion and hypoxia, causing ischemic coagulative necrosis of hepatocytes in the center of the lobule, called centrilobular necrosis - So right heart failure causes congestion, and left failure causes hypoperfusion in the liver These heart failures may only show elevated serum aminotransferases and mild jaundice o The combo of hypoperfusion and congestion work together to cause centrilobular hemorrhagic necrosis o The liver looks mottled, called “nutmeg” liver in heart failure Problems with blood flow out of the liver – will manifest as ascites, enlarged liver, abdominal pain, elevated aminotransferases, and jaundice - - Sinusoids will become dilated in any condition where blood flow out of the liver is impeded Obstruction of a single main hepatic vein is clinically silent Obstruction of 2 or more major hepatic veins causes liver enlargement, pain, and a ascites o This is called Budd-Chiari syndrome – an acute thrombosis of the major hepatic veins or liver part of the inferior vena cava o The liver damage is from increased intrahepatic blood pressure o When the inferior vena cava is obstructed near the liver part, it’s called obliterative hepatocavopathy o The liver will swell and look red-purple o The affected liver parenchyma will show severe centrilobular congestion and necrosis o There is a high mortality for untreated acute hepatic vein thrombosis Sinusoidal obstruction syndrome – happens after bone marrow transplants, and is caused by toxic injury to the sinusoid endothelium o The endothelium slough off the sinusoid wall & embolize to obstruct sinusoid blood flow o Shows tender enlarged liver, ascites, weight gain, and jaundice o Sinusoidal obstruction syndrome is characterized by obliteration of hepatic vein radicles by swelling and collagen o Will see hemosiderin deposition in liver macrophage The liver is attacked by graft-host and host-graft rxns in organ transplants - - But liver transplants are better tolerated than most other organs Acute graft-versus-host disease – donor lymphocytes attack the epithelial cells of the liver, causing hepatitis with necrosis of hepatocytes and bile duct epithelial cells, and inflammation o Takes place within 10-50 days after transplant Chronic hepatic graft-versus-host disease – 100 days or more after transplant, there’s portal tract inflammation, bile duct destruction, and fibrosis Acute rejection – shows infiltration of inflammatory cells, causing bile duct and hepatocyte injury, and endothelitis (lymphocytes lift the endothelium from its basement membrane) Chronic rejection – shows severe obliterative arteritis of small and large arteries, causing ischemia of the liver, or the bile ducts are targeted and destroyed In graft vs host disease, their stuff attacks you. In rejection, your stuff attacks their transplant Liver disease in pregnancy: - - Abnormal liver tests are seen in up to 5% of pregnancies Viral hepatitis is the most common cause of jaundice in pregnancy o HEV is more severe if the person is pregnant, otherwise pregnancy has no effect on a liver disease Less than 0.1 % of women develop liver problems because of the pregnancy: o Preeclampsia – characterized by mom hypertension, proteinuria, peripheral edema, clotting problems, and disseminated intravascular coagulation (DIC) Called eclampsia when there is hyper-reflexia and convulsion Eclampsia is life threatening Preeclampsia can show liver problems called HELLP syndrome – Hemolysis, Elevated Liver enzymes, and Low Platelets The liver in preeclampsia shows small red spots from hemorrhage The periportal sinusoids will have fibrin deposits with hemorrhage into the space of Disse This leads to periportal hepatocyte coagulative necrosis The blood pressure may cause a hepatic hematoma Dissection of blood under Glisson’s capsule may lead to catastrophic liver rupture Lab tests in preeclampsia may show elevated serum aminotransferases and serum bilirubin o Acute fatty liver of pregnancy (AFLP) – can be anywhere from slight liver dysfunction, liver failure, coma, and death More often than not though, AFLP is mild AFLP usually shows up later in pregnancy in the third trimester Symptoms are due to liver failure, including bleeding, nausea, vomiting, jaundice, and coma A lot of cases of AFLP also show preeclampsia The main treatment for AFLP is to terminate the pregnancy Fatty liver shows characteristic microvesicular fatty transformation of hepatocytes AFLP is caused by a deficiency in mitochondira long chain 3-hydroxyacy CoA dehydrogenase, so these metabolites pass from baby to mom and are toxic to her liver Liver nodular hyperlasias - noncancerous nodules on the liver - - Focal nodular hyperplasia – a single nodule on an otherwise healthy liver o Can be caused by long term use of anabolic steroids or contraceptives o Shows a central scar with fibrous tissue extending from it Nodular regenerative hyperplasia – the liver is covered by nodules, but there’s no fibrosis o Can cause portal hypertension, and happens with things that affect intrahepatic blood flow - Both nodule hyperplasias involve a change in liver blood supply Benign liver tumors: - Cavernous hemangiomas – blood vessel tumors, they look like small red-blue nodules o Most common benign liver tumor Hepatic adenoma – benign tumor developed from hepatocytes o Happen most often in women who use oral contraceptives, and the tumors regress if they stop using contraceptives o Hepatic adenomas like to rupture, especially in pregnancy when there’s high estrogen This can cause life-threatening intraperitoneal hemorrhage o Rarely, hepatic adenomas can transform into a carcinoma o Multiple hepatic adenomas (adenomatosis) can happen in maturity-onset diabetes of young (MODY3), with a mutation to HNF1 HNF1 mutations are seen in half of hepatic adenomas o Hepatic adenomas are single yellow-tan bile stained nodules found anywhere in the liver, made of cells that somewhat resemble hepatocytes Malignant liver tumors: - Primary liver tumors are rare in north America and west Europe, but pretty common elsewhere Most primary liver cancers arise from hepatocytes, & are called hepatocellular carcinoma (HCC) o HCC is the 3rd most common cause of cancer deaths o Most cases of HCC happen in developing countries with high rates of chronic HBV Half of all cases of HCC happen in china o HCC likes males more o The 4 major risk factors for HCC: Chronic HBV or HCV infection Chronic alcoholism Non-alcoholic steatohepatitis (NASH) Food contaminants – mainly aflatoxins from aspergillosis, a fungus that infects peanuts and grains a lot in china o The disease most likely to give rise to HCC is the extremely rare hereditary tyrosinemia (half of them get HCC) o In high prevalence regions, HCC is most often caused by mom giving HBV infection to the infant, greatly increasing their chances of getting HCC by adulthood Years of chronic destruction and regeneration to the liver are prime conditions for a cancer mutation to develop Cirrhosis isn’t involved in half of these cases o In the developed world, HCC almost always shows cirrhosis from a chronic liver condition o Repeated cycles of cell death and regeneration, like in chronic hepatitis, are an important cause of HCC o o o - Hepatocyte dysplasia is a precancerous sign in the hepatocyte Half of cases of HCC are associated with activation of WNT or AKT pathways Mutation to cancer may be caused by genes KRAS and p53, and expression of c-MYC, cMET (receptor for hepatocyte growth factor), TGF-α, and insulin-like growth factor 2 o HCC morphology: HCC can either be single and localized, or widely spread HCC causes the liver to enlarge HCC’s are paler than the surrounding liver, and can get a green tinge if the hepatocytes they came from could secrete bile HCC has a strong tendency to invade vasculature HCC metastasizes throughout the liver, and can then get to the portal vein or inferior vena cava o A specific kind of HCC is fibrolamellar carcinoma – happens in young people, and usually with no previous chronic liver disease, so it has a better prognosis Fibrolamellar carcinomas present as a large, hard, “scirrhous” tumor with fibrous bands through it o Often, HCC symptoms are masked by liver disease Can show upper abdominal pain, malaise, fatigue, weight loss, and abdominal fullness o 1/2 of HCCs show elevated serum α-fetoprotein, but small HCC”s often won’t show this o The natural course of an HCC is progressive enlargement of the primary mass, until it disturbs liver function, or metastasizes, usually first to the lungs o Death from an HCC is usually by cachexia (wasting away from cancer, lost weight, no appetite, etc.), GI or varices bleed, or liver failure o If it’s a large HCC, mortality is bad, with most dying within 2 years o Small HCCs can be removed by surgery with good prognosis Carcinomas of the bile duct are called cholangiocarcinomas (CCA) o Hispanics in the US are most at risk for cholangiocarcinomas o Risk factors for a CCA are primary sclerosing chalngitis (PSC), congenital polycystic diseases of the bile system, and HCV o CCA is more common in southeast Asia o CCAs can be classified as extrahepatic and intrahepatic 80-90% of CCAs are extrahepatic Klatskin (perihilar) tumor – extrahepatic CCA at the junction of the right and left hepatic ducts More than half of all CCAs are Klatskin tumors Periampullary (distal) carcinomas – CCA’s at the ampula of vater, about 1/3 of CCAs o The prognosis for CCAs is awful, with few making it 2 years o Intrahepatic CCAs aren’t usually detected until late when they obstruct bile flow They can create a tree like tumor mass in the liver bile ducts, or just a nodule - Intrahepatic CCAs often invade vasculature and lymphatics, causing lots of metastasis The tumor is very firm and gritty from dense collagen stroma o Extrahepatic CCAs show bile obstruction, cholangitis, and right upper quadrant pain Extrahepatic CCAs are small gray nodules in the bile duct wall Klatskin tumors grow the slowest of CCAs and show more fibrosis, and don’t spread often o One way a CCA can form is overexpression of Il-6, activating AKT and MCL-1 Hepatoblastoma - Most common liver tumor of children, and usually fatal within a few years if not treated o To types of hepatoblastomas: Epithelial hepatoblastoma – made of small fetal cells or embryo cells that are needed for liver development Mixed epithelial and mesenchymal hepatoblastoma – contains mesenchyme that may include osteoid, cartilage, or striated muscle o Hepatoblastomas characteristically activate the WNT/β-catenin pathway o Treatment allows a high 5 year survival rate Most liver tumors are secondary from metastasis - The liver and lungs are the organs most often targeted by metastasis