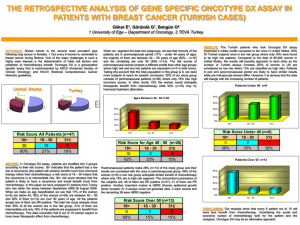
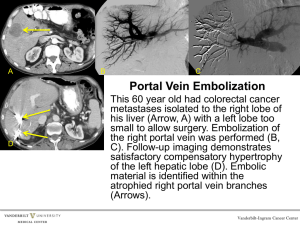
Feasibility of Surgery Chemotherapy Only in Children
advertisement

Phoenix Children’s Hospital Research Institute (PCRI) Website Clinical Trial Listing ** Please complete and return to Shy Walker at swalker@phoenixchildrens.com Study Title: Study Assessing the Feasibility of a Surgery and Chemotherapy-Only in Children With Wnt Positive Medulloblastoma Study Purpose: Participants enrolling on this study will receive standard of care chemotherapy for Wnt positive medulloblastoma without the radiation therapy or the weekly chemotherapy that is given during radiation therapy. Study Summary: There will be 9 cycles of chemotherapy. There are two different kinds of cycles given. They are referred to as A and B. Cycle A lasts for 6 weeks and Cycle B lasts for 4 weeks. B cycles are given after the completion of two A cycles. Below are the details of the drugs and schedules for A and B cycles. Cycle A (This cycle lasts 42 days) Lomustine (CCNU) is given by mouth on Day 1. Vincristine is given directly into a vein (IV) over one minute or using a minibag over several minutes by some institutions on Days 1, 8, and 15. Cisplatin is given directly into a vein over 8 hours on Day 1 Cycle B (This cycle lasts 28 days) Cyclophosphamide is given into a vein over 1 hour on Days 1 and 2. MESNA, a drug to protect the bladder from the effects of cyclophosphamide, will be given 15 minutes before each dose of cyclophosphamide and repeated at 3 and 6 hours. Vincristine is given directly into a vein directly into the vein (IV) over one minute or using a minibag over several minutes by some institutions on Days 1 and 8. You may also get a supportive care drug called a myeloid growth factor (filgrastim or pegfilgrastim). This drug will help your blood counts recover after the chemotherapy is given. Basic Eligibility Criteria: Inclusion Criteria: Participants must have classical histology posterior fossa medulloblastoma as determined by institutional neuro-pathological evaluation. Institutional beta- catenin staining must demonstrate nuclear reactivity by immunohistochemistry Sufficient pathologic material must be available for central analysis and review Tumors will be deemed Wnt positive if, at the time of central analysis, there is: Confirmation of beta-catenin nuclear reactivity by immunohistochemistry. Monosomy 6 as determined by array CGH Absence of MYCN or MYC amplification (as determined by FISH) Absence of large-cell, anaplastic histology Absence of residual or disseminated disease as defined by the following criteria: Minimal residual disease as determined by post-operative imaging preferably performed within 48 hours of resection (and at most 28 days post-surgery), i.e. gross total resection or residual disease of <1.5cm2 on postoperative imaging. No evidence of metastatic disease in the brain, spine or cerebral spinal fluid (CSF). Assessments must include MRI imaging of the brain and spine with and without contrast and a lumbar puncture for CSF cytology Patients must not have had any radiation therapy or chemotherapy for medulloblastoma prior to study enrollment Patients must have a Lansky performance status of >/=30 for children </=10 years of age or a Karnofsky performance status of > 30 for children > 10 years of age. Participants must have normal organ and marrow function as defined below: Hemoglobin greater than 10 g/dL (can be transfused). Hemoglobin <10 g/dL due to operative blood loss is permitted. Absolute neutrophil count > 1.0x109/L Platelets > 100,000/uL (non-transfused) Total bilirubin <1.5 x upper limit normal SGOT (AST) or SGPT (ALT) <2.5 x upper limit normal (ULN) for age Creatinine clearance or radioisotope GFR >70 ml/min/1.73m2 or normal serum creatinine for patient's age and gender All females of child-bearing age must have a negative pregnancy test before being enrolled on study. All patients of child-bearing age must practice an effective method of birth control whilst undergoing chemotherapy on study. No history of allergic reactions attributed to compounds of similar chemical or biologic composition to cisplatin, lomustine, vincristine or cyclophosphamide. Study Location(s): Phoenix Children’s Hospital Study Contact(s): Dianne Peterson, MS dpeterson@phoenixchildrens.com