Chapter 13
advertisement

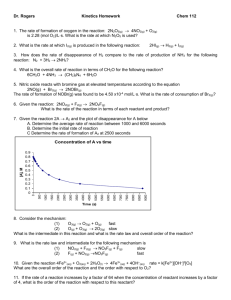
Chapter 13 RATES OF REACTION SUROVIEC SPRING 2014 I. Reaction Rates Chemical Kinetics: the investigation of the rate at which reactions occur Rate: how fast a quantity changes with time I. Reaction Rates Rate = change in quantity / time elapsed A. Average Rate Vs. Instantaneous Rate B. Writing Rate Reactions Consider the rate reaction: 2NO2 (g) 2NO (g) + O2 (g) General form aA + bB cC + dD Example Give the relative rates of disappearance of the reactants and formation of products for each of the following reactions: 2O3 (g) 3O2 (g) 2HOF (g) 2HF (g) + O2 (g) Example A 2B time [B] (mol/L) Rate (D[B]/Dt) 0.00 0.000 10.0 0.326 20.0 0.572 30.0 0.750 40.0 0.890 Rate increases or decreases as there is less A to react How is rate of change of [A] related to rate of change of [B]? Find rate of change of [A] for time internal from 10.0 to 20.0 s What is the instantaneous rate when [B] = 0.750M Example N2 (g) + 3H2 (g) 2NH3 (g) What are the relative rates? If –D[H2]/Dt = 4.5X10-4 M/min, What is –D[N2]/Dt? What is D[NH3]/Dt? II. Rate Laws Why look at initial rates? Ex. 2N2O5 4NO2 + O2 [N2O5] Rate (M/s) 0.010 0.0180 0.020 0.0360 0.040 0.0720 0.060 0.1080 0.100 0.1800 General Form of the Rate Law aA + bB cC + dD Rate = k[A]x[B]y A. Method of Initial Rates Initial rate of reaction is measured with several different starting amount of reactants Example The reaction between ozone and nitrogen dioxide at 231 K is first order in both [NO2] and [O3]. 2 NO2 (g) + O3 (g) N2O5 (g) + O2(g) Write the rate equation for the reaction 2. If the concentration of NO3 is tripled, what is the change in the reaction rates? 3. What is the effect on the reaction rate if the concentration of the O3 is halved? 1. Example 2NO(g) + O2 (g) 2NO2 (g) 1. 2. [NO] (mol/L) [O2] (mol/L) Rate of [NO] disappearing (mol/L.s) 0.010 0.010 2.5 X 10-5 0.020 0.010 1.0 X 10-4 0.010 0.020 5.0 X 10-5 3. 4. Determine the order of the reaction for each reactant Write the rate equation for the reaction Calculate the rate constant Calculate the rate in M/s at the instant when [NO] = 0.015 M and [O2] = 0.0050M Example A+BC What is the rate equation? Run # [A]i [B]i Initial Rate (M/s) 1 0.10 0.10 2.00X10-4 2 0.20 0.10 8.00X10-4 3 0.40 0.20 2.56X10-2 Base 10 log and Natural Log Base 10 log: 102 = 100 log 100 = _______ 103 = 1000 log 1000 = _______ log 45 = 1.65 101.65 =_________ log x = y 10y =x Natural Log: base is e = 2.718281828….. e1 = 2.71828 ln(2.71828) = 1 ln(e)=_______ ln(45) = 3.81 e3.81 =_________ ln x = y ey =x Important properties of both log and ln log ab = log a + log b log a/b = log a – log b log ab = b(log a) Example Rate = k[A]2[B]x III. Concentration-time relationships Useful to know How long a reaction is going to proceed to reach concentration of interest for product or reactant After a given time what are the concentrations of the reactants and the products? A. 1st order reactions AB Rate = k[A] Useful for 3 reasons Integrated rate eqn for 1st order reactions: Can calculate k if we know [A]/[A]o if k and [A]o are known then we can determine the amount of material expected after time t ([A]) If k is known than after time t we can calculate the fraction [A]/[A]o B. 2nd order reactions Rate = k[A]2 Integrated rate eqn for 2nd order reactions: 1/[A] = kt + 1/[A]o OR 1/[A] – 1[A]o = kt Example Ammonium cyanate, NH4NCO, rearranges in water to give urea, (NH2)2CO. NH4NCO (aq) (NH2)2CO (aq) The rate equation for this process is: Rate = k [NH4NCO]2 where k = 0.0113 L/mol.min. If the original concentration of NH4NCO in solution is 0.229M how long will it take for the concentration of decrease to 0.180M? Example 1st order graph y = -0.0037x - 1.1488 2 R = 0.9998 -1 ln [sucrose] Sucrose breaks down to form glucose and fructose. C12H22O11(aq) +H2O (l) 2C6H12O6 (aq) 1. Plot ln sucrose versus time and 1/[sucrose] versus time. Find the order of the reaction 2. Write the rate constant for the reaction and calculate k 3. Find concentration of sucrose After 175 min [sucrose] ln [sucrose] 1/[sucrose] 0 0.316 -1.1520131 3.164557 39 0.274 -1.2946272 3.649635 80 0.238 -1.4354846 4.2016807 140 0.190 -1.6607312 5.2631579 210 0.146 -1.9241487 6.8493151 -1.4 -1.6 -1.8 -2 0 50 100 150 200 250 time (min) + 2.9817 2nd order graph y = 0.0175x 2 R = 0.9847 1/[sucrose] time (min) -1.2 7 6.5 6 5.5 5 4.5 4 3.5 3 0 50 100 150 time (min) 200 250 C. Zero Order Reactions Rate =k[A]0 Integrated rate eqn for 0th order reactions: [A]o – [A] = kt Table 15-1, p.719 D. Half-Life Time required for half of a reactant to be consumed 1. 1st order Fig. 15-9, p.720 Example The rate equation for the decomposition of producing NO2 and O2 is –D[ N2O5]/Dt = k[N2O5] The value of k = 5.0 X 10-4 s-1 for the reaction at a known temperature. 1. Calculate the half-life of N2O5 2. How long does it take for the N2O5 concentration to drop to 1/10th of its original value? 2. 2nd order and 0th order AB AB Rate = k[A]2 Rate = k IV. Activation Energy NO2 (g) + F2 (g) FNO2 (g) + F (g) What makes this reaction occur? What makes this reaction not occur? A. Collision Theory Reactant Molecules must collide with each other Reactant molecules must collide with sufficient energy Reactant molecules must have proper orientation 2 questions: What makes a reaction go faster? What does sufficient energy mean? A. What makes a reaction go faster? 1. 2. B. What does sufficient energy mean? RXN: AB + C A + BC Mechanism: C. How can we measure Ea? From collision theory Rate of reaction = (collision freq)X(fraction with proper orientation)X (fraction of collisions with enough energy) k = Ae-Ea/RT Arrhenuis equation Example When heated to a high temperature, cyclobutane, C4H8, decomposes to ethylene. C4H8 (g) 2C2H4 (g) The activation energy, Ea for this reaction is 260 kJ/mol. At 800K the ate constant k = 0.0315 s-1. Determine the value of k at 850K. D. What is a catalyst? A substance added to a reaction to speed it up, but not consumed in the reaction MnO2 catalyzes decomposition of H2O2 2 H2O2 ---> 2 H2O + O2 Uncatalyzed reaction Catalyzed reaction V. Reaction mechanisms AB + C A + BC Possible mechanisms V. Reaction Mechanisms Molecularity Elementary step A. Mechanisms and Rate Laws 1. For net reactions, no simple relationship can be assumed between reaction coefficients and reactant orders 2. For each elementary step reactant coefficients DO equal reactant orders A. Mechanisms and Rate Laws 3. The rate of a net reaction is essentially that of the slowest step (the rate determining step) A. Mechanisms and Rate Laws 4. When a fast step proceeds a slow step the fast step can be assumed to be at equilibrium RXN: H2 (g) + CO (g) H2CO (g) Step 1. H2 (g) 2 H (g) fast Step 2. H (g) HCO (g) slow Step 3. H (g) H2CO (g) fast A. Mechanisms and Rate Laws 5. A catalyst can provide a new mechanistic pathway for a reaction 2Ce4+ (aq) + Tl+ (aq) 2Ce3+ (aq) + Tl3+ (aq) Mechanism Rate equation Add Mn2+ catalyst In General 1. Measure reaction rates 1. 2. 2. 3. Through initial rates Graphically concentraion vs. time Formulate the rate law Postulate the mechanism 1. Cannot prove, can only disprove