Chemistry Notes: Reactions, Equations, and Types
advertisement

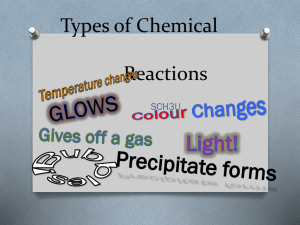
Chemistry Notes: Chapter 8 Chemical Reaction—process by which one or more substances are changed into one or more different substances. Original substances are called reactants. New substances are called products. The total mass of the reactants must always equal the total mass of the products. Chemical Equation—represents, with symbols and formulas, the identities and relative amounts of the reactants and products in a chemical reaction. There are four main indications that a chemical reaction has taken place. 1. Giving off of heat and light. 2. Production of a gas. (Bubbling) 3. Formation of a precipitate—a solid that is produced as a result of a chemical reaction in solution and that separates from the solution. 4. Changes in color. 3 Main Characteristics of a Chemical Equation. 1. The equation must represent known facts. 2. The equation must contain the correct formulas for the reactants and products. 3. The equation must satisfy the law of conservation of mass since atoms cannot be created or destroyed during a chemical reaction. a. Coefficients are added to the equation to balance the equation or satisfy the law of conservation of mass. When writing equations, certain steps must be followed. 1. First write the word equation. A word equation is an equation in which the reactants and products are represented by words. Hydrogen + oxygen water A word equation states the facts but does not specify the quantities of reactants or products. An is used to mean “yields or produces”. 2. Next, replace all the reactants and products with their correct symbols or formulas. This is called a formula equation and is it very important to get all parts of this equation written correctly. a. H2(g) + O2(g) H2O(l) b. symbols are used after each part of the equation to show the state of the substance. 3. If the equation is not balanced, it must be balanced to insure that it satisfies the law of conservation of mass. To balance an equation, coefficients are placed in front of the chemical formula so that the number of atoms of each element are equal on both sides of the equation. a. 2H2(g) + O2(g) 2H2O(l) Other symbols used by chemists—page 246. 1 A reversible reaction is a chemical reaction in which the products reform the original reactants. It is indicated with a double arrow. Chemical equations are very useful in doing quantitative chemical work because the arrow is similar to an equal sign. 1. the coefficients of a chemical reaction indicate relative, not absolute, amounts of reactants and products. 2. The relative masses of the reactants and products of a chemical reaction can be determined from the reaction’s coefficients. 3. The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction. Chemical equations do not: 1. indicate if the reaction will occur. 2. tell anything about the speed of the reaction. 3. tell anything about the bonding of atoms or ions during the reaction. 8-2 There are five main types of Chemical Reactions: 1. Synthesis or Composition a. A + X AX 2Fe(s) + O2(g) 2 FeO(s) b. Other examples: 1. Most metals react with oxygen to produce oxides. (see above) 2. Nonmetals also react with oxygen to form oxides. C(s) + O2(g) CO2(g) 3. Metals react with halogens to produce ionic or covalent compounds. 2Na+(s) + Cl2(g) 2NaCl(s) 4. Oxides of active metals react with water to produce metal hydroxides. CaO(s) + H2O(l) Ca(OH)2(s) 5. Oxides of active nonmetals react with water to produce oxyacids. SO2(g) + H2O(l) H2SO4(aq) 6. Certain metal oxides and nonmetal oxides react with each other to form salts. CaO(s) + SO2(g) CaSO3(s) 2. Decomposition Reactions—a single compound undergoes a reaction that produces two or more simpler substances. AX A + X a. Binary compounds will decompose into their elements. 2 2H2O(l) 2H2(g) + O2(g) Water will decompose into hydrogen and oxygen by passing an electric current through the water. This is called electrolysis. b. Oxides of less active metals (lower center of the periodic table) decompose into their elements when heated. 2HgO(s) 2Hg(l) + O2(g) c. Metal carbonates decompose into a metal oxide and CO2(g). CaCO3(s) CaO(s) + CO2 d. Metal hydroxides decompose into metal oxides and water. Ca(OH)2(s) CaO(s) + H2O(g) e. Metal chlorates decompose into metal chlorides and oxygen. 2KClO3(s) 2KCl(s) + 3O2(g) f. Certain acids decompose into nonmetal oxides and water. H2CO3(aq) CO2(g) + H2O(l) 3. Single replacement reactions or displacement reactions A + BX AX + B or Y + BX BY + X a. Metals can replace another metal in a compound. 2Al(s) + 3Pb(NO3)2(aq) 3Pb(s) + 2Al(NO3)3(aq) b. Metals can replace hydrogen in water. 2Na(s) + 2H2O(l) 2NaOH (aq) + H2(g) c. More active metals can replace the hydrogen in acids. Mg(s) + 2HCl(aq) H2(g)(aq) + Cl2 (s) d. More active halogens can replace less active halogens in a compound. F2(g) + 2NaCl(aq) 2NaF(aq) + Cl2(g) 4. Double replacement reactions—the ions of two compounds exchange places in an aqueous solution to form two new compounds. AX + BY AY + BX a. sometimes a precipitate forms: 2KCl(aq) + Pb(NO3)2(aq) PbI2(s) + 2KNO3(aq) b. Sometimes a gas is formed: FeS(s) + 2HCl(aq) H2S(g) + FeCl2(aq) c. Sometimes water is formed: HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) 5. Combustion reactions—when a substance combines with oxygen releasing large amounts of energy in the form of heat and light. a. C3H8(g) + 5O2 3CO2(g) + 4H2O(l) 3 8-3 Activity series—list of elements organized according to the ease with which the elements undergo certain chemical reactions. For metals—greater activity means a greater eased of loosing electrons to form + ions. For nonmetals, greater activity means a greater ease of gaining of electrons to form – ions. Any element in the series can replace the elements below it in a chemical reaction. This series can be used as a guide to predict whether certain chemical reactions will occur. Table 8-3 4